The Roots of Oral Health
Humans have been concerned with oral disease for millennia. Recent research on ancient teeth from a hunter-gatherer society reveals tooth decay from over 13,000 years ago, much before our carbohydrate and sugar rich diet 1. Other research on remains from the same time period also revealed medical intervention such as tool-assisted removal of infected or necrotic pulp, and even the application of a composite organic filling 2. Moreover, the concept of a link between oral and systemic health is ancient. Hippocrates was an early advocate of the focal infection theory that postulates that a microbial infection at one site in the body can cause disease in a distant part of the body. He was also rumored to have cured patients of rheumatism by removing infected teeth 3. More recently, and more sinisterly, some doctors at the turn of the century removed infected teeth of psychiatric patients in an effort to cure them.
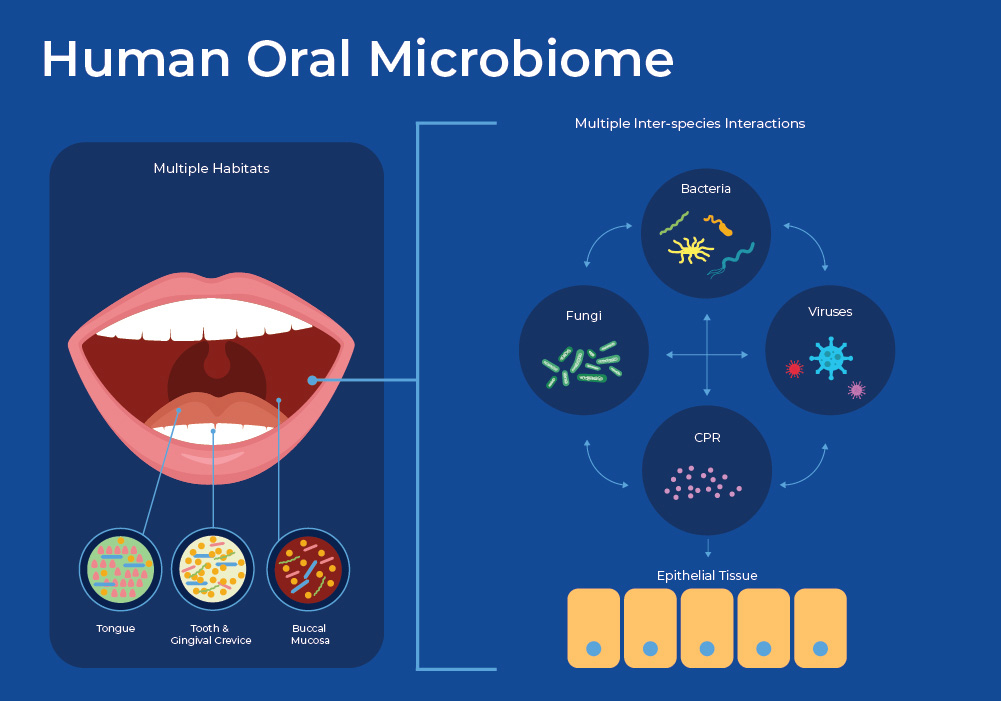
The Human Oral Microbiome
The human oral microbiome is a complex community of microbes including bacteria, viruses, bacteriophage and fungi. There have been over 700 species of oral bacteria identified, and each individual has about 250 of these living in their mouth 4. These microbes are similar in their ability to evade the anti-microbial mechanisms of saliva, and thrive in a wet, warm environment, but have a huge variety of ecological roles. Upon closer inspection, the mouth contains multiple niches including teeth, gingival sulcus, cheek, throat, tongue, and tonsils. Therefore, microbes that live within millimeters of each other can have very different characteristics. The oral microbiome, as sampled by the saliva, is then a collection of these heterogeneous niches.
Explore Saliva-Based Diagnostics

Access high-quality saliva and microbiome isolation kits tailored for innovative research and diagnostic applications.
Learn MoreOne of the more interesting findings in recent microbiology has been the discovery of a new branch of previously unidentified bacteria. This candidate phyla radiation (CPR) has been estimated to encompass 15% of microbial diversity 5. The CPR bacteria are ultramicroscopic, with reduced genomes that can only exist in a symbiotic relationship living on the surface of their bacterial host. Their inability to live independently hampers the ability to culture these bacteria. Recent estimates suggest that one group, Saccharibacteria phylum (formerly known as TM7), are ubiquitous members of the human oral microbiome and can account for up to 20% of the population. Saccharibacteria have a highly dynamic interaction with their host, and can kill or parasitize other microbes to obtain molecules that they do not produce. It is likely that Saccharibacteria influences the ecological balance of the oral microbiome by altering the overall structure, hierarchy, and functionality. 6,7
We are so reliant on our oral microbiome that we have outsourced some of our digestion capabilities to microbes. Humans do not possess the enzyme that can turn dietary nitrate to nitrite, but specific microbes in the mouth transform nitrate from fruits and vegetables into nitrite, and subsequently, nitric oxide 8. Nitric oxide is an important regulator of blood pressure. This is backed up by the fact that multiple studies have shown that using chlorhexidine containing mouthwash can have negative effects on blood pressure by causing a shift in the oral microbiome.9,10 Therefore, beyond its role in the initiation of digestion, the oral microbiome seems to be important in maintaining oral and systemic health. We will discuss how the composition of the salivary microbiome, as well as the expressed genetic material, have been shown to be important indicators of health status as well as many diseases.
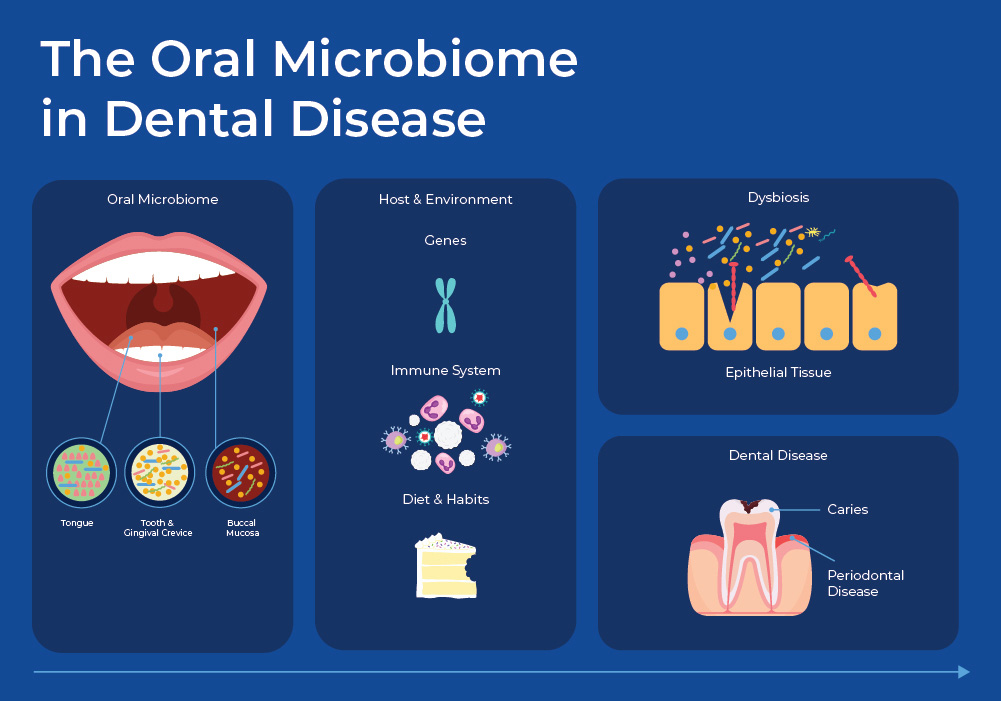
The Salivary Microbiome in Dental Disease
For decades the predominant theory was that dental diseases were caused by specific causative microbial pathogens: Streptococcus mutans for dental caries, Porphyromonas gingivalis for periodontitis, and Candida albicans for oral candidiasis. Now, culture-independent experiments combined with NGS data are showing a more nuanced picture of polymicrobial infections caused by an imbalance in the microbial communities. Opportunistic pathogens can remain neutral when kept in-balance by other microbes in the community, but when there are sudden shifts, these opportunistic pathogens can become virulent.
The Oral Microbiome and Dental Caries
Dental plaque is a term for the biofilm that exists in your mouth under normal conditions and can cover your teeth. In this biofilm, microbes have organized themselves spatially and functionally to provide a stable community that protects from pathogens. External factors such as host physiology, diet, fluoride, pH, and tooth enamel characteristics interact with this plaque biofilm to influence its homeostasis. Fermentable carbohydrates that are ingested as food are metabolized by the biofilm bacteria to produce organic acids - primarily lactic acid. These end products of bacterial metabolism accumulate in the fluid phase of the biofilm, causing a pH drop and demineralization of the surface layer of the tooth. In a biofilm under homeostasis, some microbes can reduce the chance of tooth decay (caries) by degrading lactic acid or producing a basic compound from urea and salivary peptides. But, under dysbiosis, the community shifts and other bacteria such as streptococci that lower the pH can increase the chance of caries. 11
The Salivary Microbiome in Periodontal Disease
A microbial community distinct from the tooth resides at the interface of the teeth and the host gum epithelium. When this microbiome is in dysbiosis, it can cause periodontal disease (PD). PD is an inflammatory disease where gum inflammation can progress to tooth loss and bone loss. The healthy gingival community contains a symbiotic mixture including many facultative bacteria such as Actinomyces and Streptococcus. However, dysbiosis can cause a shift to mainly anaerobic types, such as the Firmicutes, Proteobacteria, Spirochaetes, Bacteroidetes, and Synergistete. Specific opportunistic pathogens that have been identified include P. gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Prevotella intermedia, and Fusobacterium nucleatum. Interestingly, the fungus Candida albicans has also been shown to play an indirect role by shielding P. gingivalis from the host's immune system.13
Recent research has revealed one mechanism by which a community can shift from healthy to diseased that involves cross-species interaction with a symbiotic commensal. The opportunistic pathogen P. gingivalis is dependent on autoinducing molecules to grow, so when the population density is low the molecule is not produced, and thus its population growth is limited. Researchers used in vitro culture systems, as well as a disease-relevant mouse model, to show that an early colonizer, Veillonella parvula, supports growth of a low-cell-density inoculum of P. gingivalis through releasing a soluble molecule 14. High numbers of V. parvula were needed to reach a threshold concentration before it could induce P. gingivalis growth. This suggests that once a healthy biofilm reaches a certain population number, there is enough V. parvula to support the excessive growth of P. gingivalis, with the increased population density leading to pathogenesis.
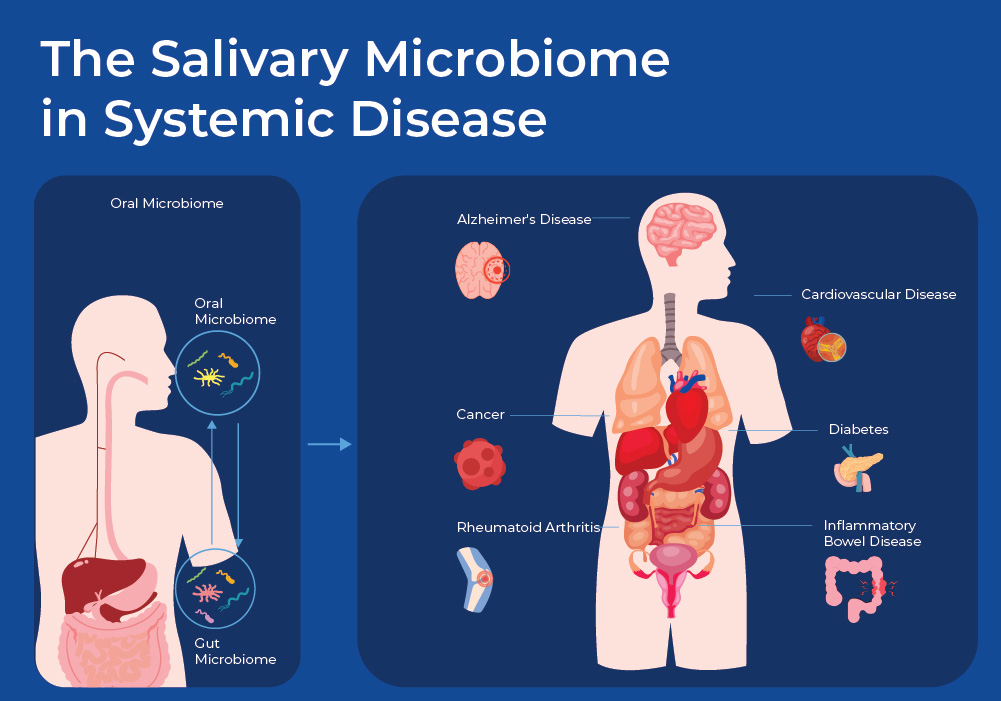
The Salivary Microbiome in Systemic Disease
With saliva being a sample that is easy to collect, the oral microbiome is one of the best studied microbiomes. Studies have now found links between the oral microbiome and a multitude of diseases, such as inflammatory bowel disease, rheumatoid arthritis, cardiovascular disease, Alzheimer’s disease, diabetes, pre-term birth, as well as pancreatic and colon cancer. As researchers are finding consistent trends in the change of the oral microbiome, the hope is that the oral microbiome can be used to predict disease, as well as to monitor different treatments. 15
There are both direct and indirect routes by which bacteria can have systemic effects across the body. One direct route involves swallowing bacteria contained in saliva. Estimates suggest that every day, humans swallow around 1.5 liters of saliva that contain about 108–1010 microbes. Several mechanisms exist that limit the number of live microbes that make it to the gut, including the low acidity of the stomach and bile acids in the duodenum; however, evidence suggests that there is continual transfer from oral to gut. Another direct route by which oral bacteria can travel through the rest of the body is through the microvasculature in the gums and through tight junctions 16. In addition to direct transfer of bacteria into the vascular system, bacterial products such as LPS get transported through the body. LPS of P. gingivalis has been shown to bind to Toll Like Receptor 4 (TL-4) on the host cell membrane. This then activates the NF-κB signaling pathway, leading to the production of inflammatory cytokines, such as Tumor Necrosis Factor α (TNF-α) and Interleukin 6 (IL-6), and chemokines, such as Monocyte Chemoattractant Protein 1 (MCP-1)17. In this blog we will discuss how oral microbes can impact two specific types of systemic diseases.
Oral Microbiome and Cardiovascular Health
Globally, cardiovascular diseases (CDs) claim the lives of an estimated 17.9 million individuals each year, making them the primary cause of death. Therefore, any efforts to use the oral microbiome as an early warning-signal, or to monitor treatment, could have a positive impact. Many large studies have revealed a link between Periodontal Disease (PD) and Cardiovascular Disease. For example, in a large study involving over 1600 patients, the risk of a first myocardial infarction was significantly increased in patients with PD. Due to the large number of patients in this study, the results were rigorously controlled for possible confounding factors such as smoking, medications, and other factors.18 These associative studies are also backed up by more mechanistic studies.
In a recent study, hospitalized patients were analyzed for cardiac biomarkers, and metagenomic and metatranscriptomic analysis was performed on subgingival plaque and coronary thrombi. Cardiac trauma, as measured by Troponin-I, increased with multiple markers of periodontal disease, such as inflamed surface area, probing pocket depth, and clinical attachment loss. Moreover, levels of P. gingivalis, as measured by 16S copy numbers, increased with myocardial injury and coronary artery disease burden. Gene expression analysis confirmed that upregulation of virulence genes also correlated with increased myocardial injury and coronary artery disease burden. The upregulated genes are involved in pathogenic phenotypes. For example, bacterial adhesion necessary for intracellular and transepithelial invasion (finA of P. gingivalis), in mounting pro-inflammatory responses via TLR ( bioF-3 for P. gingivalis), and degradation of intercellular adhesins facilitating bacterial invasion beyond the epi/endothelium barrier host-defense evasion (well as the prtH and prtP genes of T. forsythia, T. denticola). Lastly, they found physical evidence of bacterial translocation, as a correlation was observed between bacterial loads detected in coronary thrombi and corresponding subgingival plaque from the same patient for P. gingivalis and T. forsythia19.
A new immunoprofiling technique was used to investigate the impact of the oral microbiome on the immune system in the context of Cardiovascular Disease (CD). Mimotope Variation Analyses (MVA) is a novel technique that combines phage display of 12-mer peptide antigens called mimotopes with NGS to generate quantitative serologic profiles. A recent study used MVA to profile 96 individuals with and without CD, classified by their periodontal health. Peptide antigens that were captured by the phage display library were then sequenced to identify peptide epitopes related to the antibody immune response in periodontal disease and CAD. By mapping the 8088 epitope sequences onto proteomes, they showed immunoreactivity to the 7 most common periodontal pathogens, and identified a strong antibody response to P. ginivalis. Moreover, they identified some dominant shared core epitopes. There was a strong response to epitope A with the core pattern P..T.PR, which has been previously mapped to the Epstein Barr Virus (EBV VP26). They observed that subjects within the periodontitis group exhibited high immunoreactivity to this C-terminal epitope. Furthermore, multivariable models were fit using the antibody response to epitopes, and they can stratify subjects with periodontitis from healthy controls according to their antibody response to EBV, in particular to VP26 and sequence-mimicking bacterial antigens.20
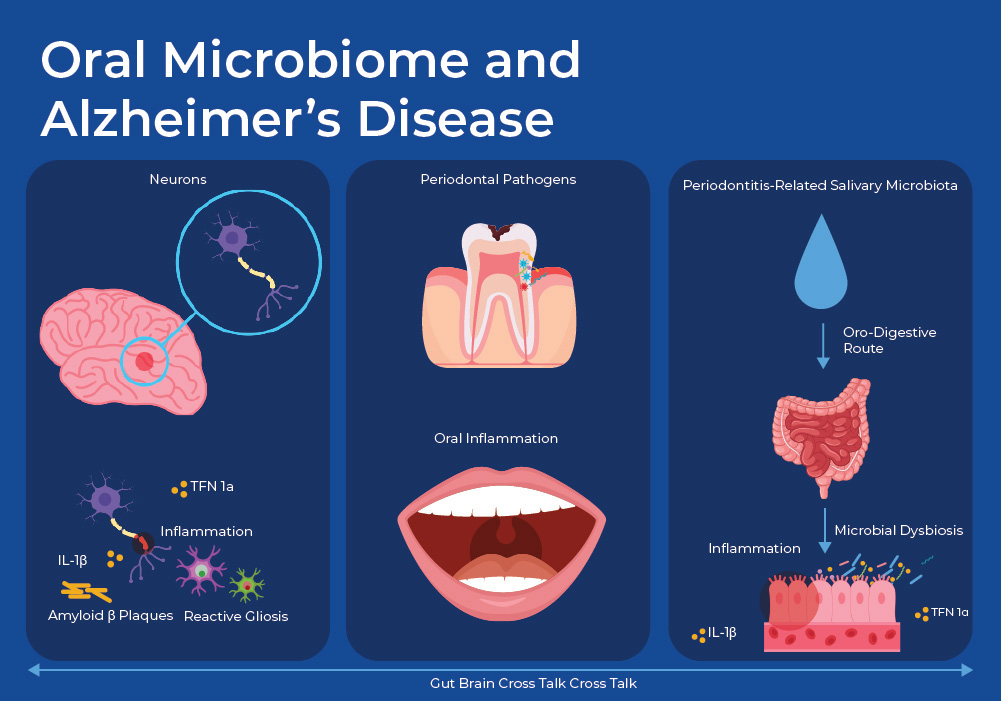
Oral Microbiome and Alzheimer's Disease
A growing body of evidence suggests that the presence of periodontal pathogens and the ensuing inflammatory response plays a role in the development of neurodegenerative diseases such as Alzheimer’s Disease (AD). A recent mouse study revealed mechanisms of this link. They gavaged mice with either oral microbes from subjects with periodontal disease (PD), or healthy subjects. First, using 16S metagenomic sequencing they confirmed that the salivary microbiome differs significantly between the healthy subjects and those with PD. About 90 % of the species, as identified by ASVs, were unique in the PD subjects, with known pathogens such as Treponema, Porphyromonas, and Fusobacterium being enriched. Secondly, they showed that the mice that were gavaged with PD saliva showed increased levels of anxiety and decreased cognitive function. They then showed that levels of Aβ oligomers and pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin (IL)-1β, were significantly more abundant in the cortex of the mice gavaged with PD saliva as compared to those gavaged with healthy saliva.
In addition to examining AD biomarkers in the brain, they looked at the effect of the PD gavage on the intestines and the gut microbiome. The level of fecal lipocalin-2 (LCN2), which promotes intestinal inflammation, was higher in PD-gavaged mice. Moreover, RNA sequencing showed that periodontitis-related salivary microbiota treatment significantly changed immune response-related gene expression in the intestine, with upregulation of cytokine genes (such as TNF-α, IL-B) in the colon tissue. Other evidence pointed to the oral microbiome having a negative impact on the intestinal integrity, such as reduced expressions of tight junction-related proteins ZO-1 and occludin, which play an essential part in maintaining the intestinal barrier, and increased content of fecal albumin. Additionally, staining showed the damage to intestinal mucus layer in the PD group, with a decreased number of goblet cells in each crypt. ELISA analysis showed that the plasma concentrations of TNF-α and IL-1β, which reflected the level of systemic inflammation, were significantly increased in mice treated with periodontitis-related salivary microbiota compared to those in healthy mice 21 . Together these results link PD-associated oral microbes with increased amounts of biomarkers of AD and inflammation, linked to the gut through increased biomarkers of intestinal inflammation and reduced tight junction integrity.
The Future of Saliva as a Tool for Diagnostic and Treatment Decisions
Saliva is an abundant biological fluid that can be collected non-invasively. The insights gained from its analysis can complement clinical and histopathological findings in diagnosing a variety of diseases. As sequencing methods improve, new metagenomic and metatranscriptomic data will help reveal microbial trends with more diseases. As we learn more about the mechanisms behind these diseases, the composition of the oral microbiome can be used for early diagnosis, as well as to monitor treatment progress. Moreover, novel techniques are emerging to modulate the dysbiotic oral microbiome. These include prebiotics and postbiotics, specific antimicrobial peptides, compounds to degrade EPS, and host response modulators.
Norgen's Saliva Microbiome Products
Norgen offers a complete Saliva workflow, starting from preservation devices all the way to NGS sequencing services. The Saliva Collection and Preservation devices inactivate microbes and maintain the integrity of the nucleic acids at room temperatures. A variety of extraction kits for both Saliva DNA and Saliva RNA are available in multiple formats, including those using magnetic beads or 96-well plates for high throughput options.