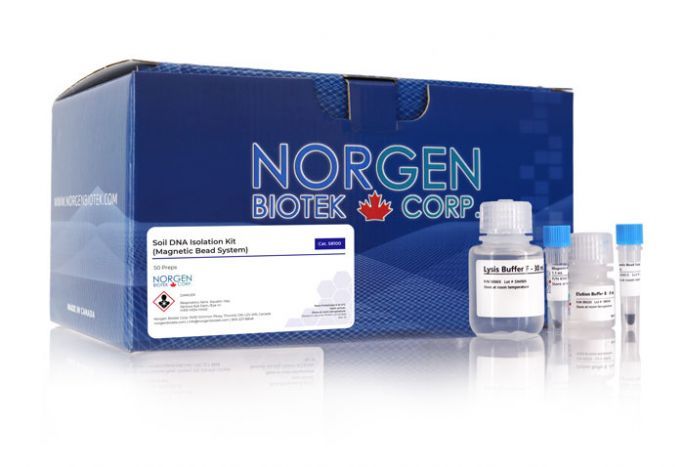
Soil DNA Isolation Kit (Magnetic Bead System)
For isolating genomic DNA from soil samples
For research use only and NOT intended for in vitro diagnostics.
For isolating genomic DNA from soil samples
For research use only and NOT intended for in vitro diagnostics.
For isolating genomic DNA from soil samples
Register today to receive an exclusive 15% off* on your first order.
Norgen’s Soil DNA Isolation Kit (Magnetic Bead System) provides a fast and reproducible method for isolating genomic DNA from soil samples. All types of soil samples can be processed with this kit, including common soil samples and difficult soil samples with high humic acid content such as compost and manure. The kit removes all traces of humic acid using the provided the OSR (Organic Substance Removal) Solution. Total genomic DNA can be isolated and purified from all the various microorganisms found in soil, such as bacteria, fungi and algae. The purified DNA is of the highest quality and is fully compatible with downstream PCR applications, as all humic acid substances and PCR inhibitors are removed during the isolation.
Norgen's Soil DNA Isolation Kit (Magnetic Bead System) is also available in a 96-well format for high throughput applications. Purification with the 96-well plates can be integrated with a robotic automation system.
Figure 1. Resolution of DNA isolated from two different types of soil samples DNA was isolated from high humic acid soil (top soil) and regular soil (clay) using Norgen’s column-based Soil DNA Isolation Kit (Red C) and Norgen's Soil DNA Isolation Kit (Magnetic Bead System) (Mag Bead). For evaluation, 10 µL from the 75 µL elution was run on 1X TAE 1.2% agarose gel. Excellent DNA integrity and yield were observed from the Soil DNA Isolation Kit (Magnetic Bead System), indicating the robust performance comparable to the column based method. Marker = Norgen’s HighRanger DNA Ladder.
Figure 2. Two of Norgen’s soil DNA isolation methods (Column vs Magnetic Bead System) were compared for DNA concentration. All DNA elutions isolated using Norgen’s Soil DNA Isolation Kit (Magnetic Bead System) [A to E] showed a comparable DNA concentration to Norgen’s Soil DNA Isolation Kit (Column Method; Cat. 26500), indicating the consistent and robust performance of the Soil DNA Isolation Kit (Magnetic Bead System).
Figure 3. High soil DNA quality was confirmed by real-time PCR using 8 µL of soil DNA (total PCR reaction volume was 20 µL) isolated using Norgen’s Soil DNA Isolation Kit (Magnetic Bead System) to detect 16s rDNA. PCR results from Norgen's Soil DNA Isolation Kit (Magnetic Bead System) (circle) were comparable to Norgen’s column based Soil DNA Isolation Kit (cross) and showed the successful 16s rDNA detection without PCR inhibition, indicating the excellent soil DNA quality using Norgen’s Soil DNA Isolation Kit (Magnetic Bead System).
Figure 4. Hierarchical Clustering Dendrogram. High quality of soil DNA was successfully isolated from the 5 challenging soil types using Norgen's Soil DNA Isolation Kits (A: Soil DNA Isolation Kit (Magnetic Bead System) and B: Soil DNA Isolation Kit Spin Column) respectively. The Hierarchical Clustering Dendrogram is based on genus-level classifications and shows the relative abundance of its genus-level classifications among soil types.
Figure 5. Principal Coordinate Analysis (PCoA) generated by Illumina MiSeq. Principal Coordinate Analysis of 20 samples (25-28: Mulch, 29-32: Clay, 33-36: Potting soil, 76-78: Top soil, 79-82: Compost) showing differences in the distribution of taxonomic classifications between samples up to species level. Each soil type is clearly clustered together and away from other types.
Figure 6. Resolution of DNA Isolated from Top Soil. DNA was isolated from high humic acid soil (top soil) using Norgen's Soil DNA Isolation 96-Well Kit (Magnetic Bead System). For evaluation, 10 µL from the 75 µL elution was run on 1X TAE 1.2% agarose gel. Excellent DNA integrity, consistency and yield were observed from the Soil DNA Isolation 96-Well Kit (Magnetic Bead System). Marker = Norgen’s HighRanger DNA Ladder (Cat. 11900).
Figure 7. High Soil DNA Quality Was Confirmed By Real-time PCR Using 2 µL of Soil DNA (Total PCR Reaction Volume Was 20 µL) Isolated Using Norgen’s Soil DNA Isolation 96-Well Kit (Magnetic Bead System) to Detect 16s rRNA. PCR results from Norgen's Soil DNA Isolation 96-Well Kit (Magnetic Bead System) showed the successful 16s rRNA detection without PCR inhibition, indicating the excellent soil DNA quality using Norgen’s Soil DNA Isolation 96-Well Kit (Magnetic Bead System).
|
Kit Specifications
|
|
|
Maximum Soil Input
|
0.25 g
|
|
Type of Soil Processed
|
All types of soil
|
|
Average Yield from 0.25 mL of Soil*
|
1 - 5 μg
|
| Average Purity (OD260/280) |
1.7 - 1.8
|
| Time to Complete Purifications |
40 minutes (Cat. 58100)
50 minutes (Cat. 62800) |
* Average DNA yields will vary depending upon soil sample types
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 1 year after the date of shipment.
| Component | Cat. 58100 (50 preps) | Cat. 62800 (2 x 96-well) |
|---|---|---|
| Lysis Buffer G | 100 mL | 1 x 100 mL 1 x 45 mL |
| Lysis Additive A | 6 mL | 25 mL |
| Binding Buffer I | 7 mL | 25 mL |
| OSR Solution | 3 mL | 12 mL |
| Binding Buffer B | 2 x 12 mL | 85 mL |
| Magnetic Bead Suspension | 2 x 1.1 mL | 8.5 mL |
| Buffer SK | 30 mL |
1 x 40 mL 1 x 60 mL |
| Elution Buffer B | 8 mL | 15 mL |
| Elution Tubes (1.7 mL) | 50 | - |
| Bead Tubes | 50 | 196 |
| 96-Well Plate | - | 2 |
| 96-Well Elution Plate | - | 2 |
| Adhesive Tape | - | 2 |
| Product Insert | 1 | 1 |
If the magnetic beads were accidently pipetted up with the supernatant, the pipette tip was placed too close to the magnetic beads while pipetting. Simply return the magnetic beads and the supernatant back into the sample well. Mix well, and place the plate back onto the magnetic separation plate for the specified time. Carefully remove the supernatant without touching the magnetic beads
A low genomic DNA yield may be caused by the following:
Ensure that Lysis Additive A is added. Also, incubation at 65ºC may result in increased yields.
Ensure that the magnetic bead suspension is mixed well prior to use to avoid any inconsistency in DNA isolation.
Some soil types contain very little target DNA. Incubation at 65ºC may result in increased yields.
If the DNA does not perform well in downstream applications, it may be due to one or more of the following:
The elution contains high humic acids. Ensure that the OSR Solution was added to the clean lysate.
Traces of humic acids or salt from the binding step may remain in the sample if the magnetic beads are not washed with the provided Buffer SK. Humic acids and salt may interfere with downstream applications and thus must be washed from the magnetic beads.
Ensure that the drying step after the 70% ethanol wash steps is performed in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
Ran can get co-eluted with DNA. Carry out a digestion with RNase A on the elution if the RNA present will interfere with downstream applications. Refer to manufacturer’s instructions regarding amount of enzyme to use, optimal incubation time and temperature.
| Title | Arbuscular mycorrhizal fungal diversity and potential association networks among African tropical forest trees |
| Citation | Mycorrhiza 2024. |
| Authors | Damilola Olanipon, Margaux Boeraeve & Hans Jacquemyn |
| Title | Symbiotic microbiota vary with breeding group membership in a highly social joint-nesting bird |
| Citation | Behavioral Ecology 2023. |
| Authors | Leanne A Grieves, Gregory B Gloor, James S Quinn |
| Title | Acclimatization of a mixed-animal manure inoculum to the anaerobic digestion of Axonopus compressus reveals the putative importance of Mesotoga infera and Methanosaeta concilii as elucidated by DGGE and Illumina MiSeq |
| Citation | Bioresource Technology 2017. |
| Authors | Lee, J. T., He, J., & Tong, Y. W. (2017). |
| Title | Selection of a Very Active Microbial Community for the Coupled Treatment of Tetramethylammonium Hydroxide and Photoresist in Aqueous Solutions |
| Citation | International Journal of Environmental Research and Public Health 2017. |
| Authors | Moretti, G., Matteucci, F., Saraullo, M., Vegliò, F., & del Gallo, M. (2017). |
| Title | It takes an individual plant to raise a community: TRFLP analysis of the rhizosphere microbial community of two pairs of high-and low-metal-accumulating plants |
| Citation | Soil Biology and Biochemistry 2014. |
| Authors | MP Columbus, SM Macfie |