Cells and Tissue DNA Isolation Kits
For the isolation of genomic DNA from various types of animal tissues or cell samples
For research use only and NOT intended for in vitro diagnostics.
Cells and Tissue DNA Isolation Kits
For the isolation of genomic DNA from various types of animal tissues or cell samples
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Fast, reproducible and easy processing of samples
- High yield and high quality genomic DNA with no RNA or protein contamination
- DNA ready for any application including PCR, qPCR, genotyping and more
Norgen’s Cells and Tissue DNA Isolation Kits are designed for the rapid preparation of genomic DNA from cultured cells as well as various tissue samples and urine. The purified genomic DNA is fully digestible with all restriction enzymes tested, and is completely compatible with PCR and Southern Blot analysis.
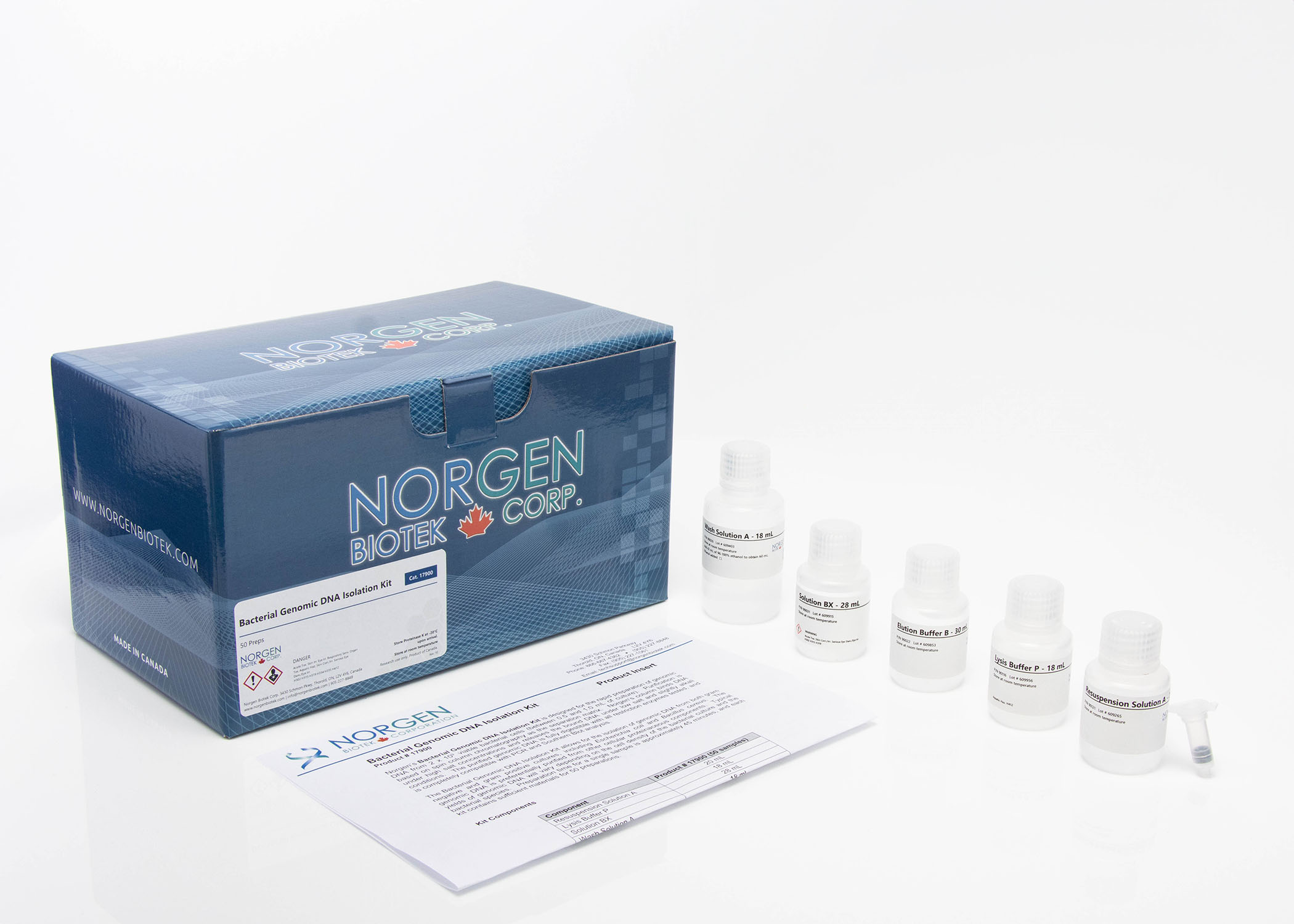
Cells and Tissue DNA Isolation Kit (Spin Column)
Purification is based on spin column chromatography as the separation matrix. Norgen’s columns bind DNA under optimized salt concentrations and release the bound DNA under low salt and slightly alkali conditions. The protocol can be completed in approximately 30 minutes for cells and within 90 minutes for tissues. Each kit contains sufficient materials for 50 preparations.
Cells and Tissue DNA Isolation Micro Kit (Micro)
Optimized for small inputs of cells and tissues, such as Laser-Captured Microdissection (LCM). Purification is based on spin column chromatography as the separation matrix. Norgen’s columns bind DNA under optimized salt concentrations and release the bound DNA under low salt and slightly alkali conditions. Preparation time for a single sample is approximately 60 minutes, and each kit contains sufficient materials for 50 preparations.
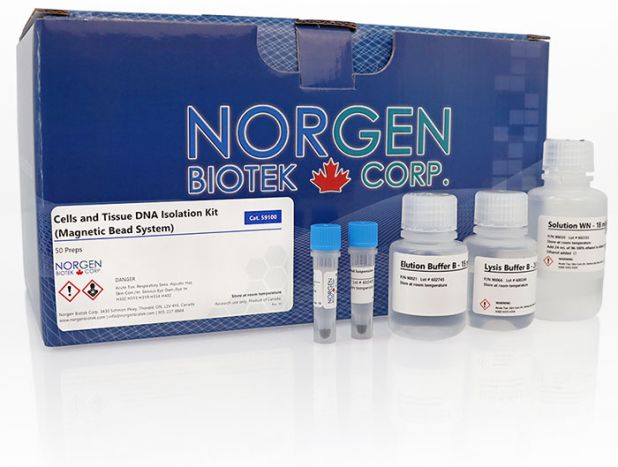
Cells and Tissue DNA Isolation Kit (Magnetic Bead System)
Purification is based on the use of magnetic beads that bind DNA under optimized binding conditions. Norgen’s Cells and Tissue DNA Isolation Kit (Magnetic Bead System) allows for the isolation of genomic DNA from various types of animal tissues or cell samples. Preparation for 50 purifications is approximately 40 minutes of hands-on time, or just 15 minutes with automation.
Cells and Tissue DNA Isolation 96-Well Kit (High Throughput Magnetic Bead System)
Purification is based on the use of magnetic beads that bind DNA under optimized binding conditions. Norgen’s Cells and Tissue DNA Isolation 96-Well Kit (Magnetic Bead System) allows for the isolation of genomic DNA from various types of animal tissues or cell samples. The Cells and Tissue DNA Isolation 96-Well Kit (Magnetic Bead System) also can be integrated with a robotic automation system.
Details
Supporting Data
Figure 1. Isolation of High Quality Genomic DNA from Tissue. Genomic DNA was isolated from 15 mg of liver tissue using Norgen's Cells and Tissue DNA Isolation Kit. Triplicate samples were used (lanes 1-3) and yielded genomic DNA of the highest quality and integrity. Lane M is the Norgen UltraRanger 1kb DNA Ladder. For all purified DNA, 15 µL of each 200 µL elution were resolved on a 1X TAE, 1% agarose DNA gel.
Figure 2. Isolation of High Quality Genomic DNA from 0.1 up to 3 million mammalian cells. Genomic DNA was isolated from 0.1 x 106 to up to 3 million HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit. Triplicate samples were used from each cell input and yielded genomic DNA of the highest quality and integrity with linear increase in yield with increasing cell input. Lane M is the Norgen UltraRanger 1kb DNA Ladder. For all purified DNA, 15 µL of each 200 µL elution were resolved on a 1X TAE, 1% agarose DNA gel.
Figure 3. Yield of Genomic DNA from 0.1 x 106 up to 3 x 106 mammalian cells. Genomic DNA was isolated from 0.1 up to 3 million HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit. Triplicate samples were used from each cell input and 25 µL of the 200 µL elution were diluted in 475 µL of nuclease-free water, and DNA concentrations were measured using the UltraSpec 2100 Pro (Fisher Scientific). Linear increase in DNA yield was obtained when increasing the cell input from 0.1 x 106 - 3 x 106 HeLa cells.
Figure 4. Purified DNA Can be Amplified in a Real-time PCR (TaqMan) Reaction. Genomic DNA was isolated from 0.1 x 106 to 3 x 106 of HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit. Five µL of the DNA from each 200 µL elution was used in a real-time PCR reaction (total reaction volume of 20 µL) with GAPDH TaqMan probe and primers. The real-time PCR was successful in amplifying the GAPDH gene from all the isolated DNA from the different cell inputs with a decreasing Ct value with increasing the cell input. This indicates that the kit provides high quality DNA in each case and is free of PCR inhibitors making it well suited to sensitive downstream applications.
Figure 5. Resolution of DNA isolated from Animal Tissue and Mammalian Cells. DNA was isolated from 20 mg animal tissue (beef) and 1 x 106 HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit (Magnetic Bead System), and Norgen's Cells and Tissue DNA Kit (column format, Cat. 53100) as a control. The DNA isolated with the Magnetic Bead System (B) was then compared with the column method (C). For evaluation, 10 µL from each 200 µL of elution were run on 1X TAE 1.2% agarose gel. As it can be seen, Norgen's Magnetic Bead System (B) was able to isolate high yields of DNA from both the tissue and cells. M = Norgen's HighRanger DNA Ladder.
Figure 6. Comparison of A260/280 and A260/230 ratios. DNA was isolated from 20 mg animal tissue (beef) and 1 x 106 HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit (Magnetic Bead System), and Norgen's Cells and Tissue DNA Kit (column format, Cat. 53100). The DNA isolated with the Magnetic Bead System was then compared with the column method for A260/280 and A260/230 rations. DNA isolated using Norgen's Cells and Tissue DNA Isolation Kit (Magnetic Bead System) showed a comparable DNA quality to the column method (Cat. 53100).
Figure 7. High Quality DNA was confirmed by Real-time PCR. DNA was isolated from 20 mg animal tissue (beef) and 1 x 106 HeLa cells using Norgen's Cells and Tissue DNA Isolation Kit (Magnetic Bead System). The high quality of the purified DNA was confirmed by Real-time PCR using 2 µL (circle), 4 µL (triangle) and 8 µL cross) of animal tissue DNA or HeLa DNA (total PCR reaction volume was 20 µL) to detect 5s rDNA or GAPDH from animal tissue and HeLa cell respectably. No PCR inhibition was observed from the all different PCR template amounts, indicating the excellent quality of animal tissue and HeLa DNA for downstream applications.
Figure 8. DNA was Isolated from 1 x 106 HeLa Cells Using Norgen's Cells and Tissue DNA Isolation 96-Well Kit (Magnetic Bead System). For evaluation, 10 µL from each 100 µL of elution were run on 1X TAE 1.2% agarose gel. As it can be seen, Norgen's Cells and Tissue DNA Isolation 96-Well Kit (Magnetic Bead System) was able to isolate consistent and high yields of DNA from HeLa cells. M = Norgen's HighRanger DNA Ladder (Cat. 11900).
Figure 9. High Quality DNA was Confirmed by Real-Time PCR. DNA was isolated from 1 x 106 HeLa cells using Norgen's Cells and Tissue DNA Isolation 96-Well Kit (Magnetic Bead System). The high quality of the purified DNA was confirmed by Real-time PCR using 2 µL of HeLa DNA (total PCR reaction volume was 20 µL) to detect GAPDH from HeLa cell. No PCR inhibition was observed indicating the excellent quality of HeLa DNA for downstream applications.
|
Kit Specifications
|
|
| Maximum Input |
20 mg of animal tissue |
| Column Binding Capacity | > 50 μg |
| Average Yield | 8 μg (from 1 x 106 HeLa Cells) 10 μg (from 10 mg kidney) |
| Elution Volume | 50 - 200 μL |
| Analyte Purified | Genomic DNA, mitochondrial DNA, viral DNA |
| Format | Spin Column |
| Time to Complete 10 Purifications | 30 min (cells) and 90 min (tissue) |
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 1 year after the date of shipment. The kit contains a ready-to-use Proteinase K, which is dissolved in a specially prepared storage buffer. The buffered Proteinase K is stable for up to 1 year after the date of shipment when stored at room temperature.
| Component | Cat. 53100 (50 preps) | Cat. 57300 (50 preps) | Cat. 59100 (50 preps) | Cat. 62500 (192 preps) |
|---|---|---|---|---|
| Lysis Buffer B | 20 mL | 20 mL | 20 mL | 1 x 40 mL 1 x 20 mL |
| Solution WN | 18 mL | 18 mL | 18 mL | 55 mL |
| Wash Solution A | 18 mL | 18 mL | - | - |
| Elution Buffer B | 30 mL | 8 mL | 15 mL | 1 x 30 mL 1 x 15 mL |
| Proteinase K | 1.2 mL | 1.2 mL | - | - |
| Proteinase K in Sotrage Buffer | - | - | 1.2 mL | 4 mL |
| Magnetic Beads A | - | - | 2 .2 mL | 8.5 mL |
| 96-Well Plate (Manual Extraction) | - | - | - | 2 |
| Spin Columns | 50 | - | - | - |
| Micro Spin Columns | - | 50 | - | - |
| Collection Tubes | 50 | 50 | - | - |
| Elution Tubes (1.7 mL) | 50 | 50 | 50 | - |
| 96-Well Elution Plate (Manual Extraction) | - | - | - | 2 |
| Adhesive Tape | - | - | - | 2 |
| Product Insert | 1 | 1 | 1 | 1 |
Documentation
(57300) Cells and Tissue DNA Isolation Micro Kit - Protocol (50 preps)
(59100) Cells and Tissue DNA Isolation Kit (Magnetic Bead System) - Protocol (50 prep)
(62500) Cells and Tissue DNA Isolation 96-Well Kit (High Throughput Magnetic Bead System) - Protocol (2 x 96-well)
Comparing Different DNA and RNA Quantification Methods for Biological Samples with Low Nucleic Acid Abundance
Determination of the DNA Molecular Weight (MW) from different Norgen Columns and Isolation Methods
FAQs
Spin Column
Column clogging can result from the following:
- The sample is too large.
Do not exceed the recommended amount of starting materials. The amount of starting material may need to be decreased if the column shows clogging below the recommended levels. Clogging can also be alleviated by increasing the g-force and/or centrifuging for a longer period of time until the lysate passes through the column.
Lysate can be more gelatinous prior to loading onto the column due to the following factors:
- The lysate solution mixture is not homogeneous.
To ensure a homogeneous solution, vortex for 10-15 seconds before applying the lysate to the spin column. - Maximum number of cells or amount of tissue exceeds kit specifications.
Refer to specifications to determine if the amount of starting material falls within kit specifications.
A low genomic DNA yield may be caused by:
- Improper storage of samples.
Tissue samples and cell pellets may be frozen and stored at -20°C or -80°C. Repeated freezing and thawing of stored samples should be avoided, as this may lead to decreased yields of DNA. - Incomplete lysis of cells.
Ensure efficient homogenization of tissue samples and extend the incubation time of Proteinase K digestion or reduce the amount of tissue or cells used for lysis.
Shearing of genomic DNA can be due to the following reasons:
- The genomic DNA was handled improperly.
Pipetting steps should be handled as gently as possible. Reduce vortexing times during mixing steps (no more than 10-15 seconds). - Improper storage of sample.
Repeated freezing and thawing of stored samples should be avoided as this may lead to decreased DNA size. - The sample is old.
Sheared DNA may be obtained from old tissue or cell samples. Fresh samples are recommended for best genomic DNA yield.
If the DNA does not perform well in downstream applications, it may be due to one or more of the following:
- DNA was not washed with the provided solutions.
Ensure the column was washed once with Wash Solution WN and twice with Wash Solution A. - Ethanol carryover.
Ensure that the dry spin under the Column Wash procedure is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
Yes, Product number 28229 is the correct replacement part for the kits tagged in "Products". It comes with 5 tubes of 1 ml each.
Yes, it is possible to isolate bacterial DNA present in tissue samples using 53100 kit. Just add 6 mg of Lysozyme (15 µL from 400 mg/mL stock solution) along with Proteinase K to the lysate during the incubation step.
Yes, it is possible to isolate DNA from bone tissue using this kit after decalcification of the tissue. Please feel free to contact our Tech support team at support@norgenbiotek.com for help with decalcification protocol.
Yes, it is possible to isolate DNA from insect samples using this kit. For large specimens, consider dissecting the insect to remove exoskeleton. For smaller specimens, grind the sample with liquid nitrogen using pestle and mortar. Please contact our Tech support team at support@norgenbiotek.com and ask for reference publications.
Micro
Column clogging can result from the following:
- The sample is too large.
Do not exceed the recommended amount of starting materials. The amount of starting material may need to be decreased if the column shows clogging below the recommended levels. Clogging can also be alleviated by increasing the g-force and/or centrifuging for a longer period of time until the lysate passes through the column.
Lysate can be more gelatinous prior to loading onto the column due to the following factors:
- The lysate solution mixture is not homogeneous.
To ensure a homogeneous solution, vortex for 10-15 seconds before applying the lysate to the spin column. - Maximum number of cells or amount of tissue exceeds kit specifications.
Refer to specifications to determine if the amount of starting material falls within kit specifications.
A low genomic DNA yield may be caused by:
- Improper storage of samples.
Tissue samples and cell pellets may be frozen and stored at -20°C or -80°C. Repeated freezing and thawing of stored samples should be avoided, as this may lead to decreased yields of DNA. - Incomplete lysis of cells.
Ensure efficient homogenization of tissue samples and extend the incubation time of Proteinase K digestion or reduce the amount of tissue or cells used for lysis.
Shearing of genomic DNA can be due to the following reasons:
- The genomic DNA was handled improperly.
Pipetting steps should be handled as gently as possible. Reduce vortexing times during mixing steps (no more than 10-15 seconds). - Improper storage of sample.
Repeated freezing and thawing of stored samples should be avoided as this may lead to decreased DNA size. - The sample is old.
Sheared DNA may be obtained from old tissue or cell samples. Fresh samples are recommended for best genomic DNA yield.
If the DNA does not perform well in downstream applications, it may be due to one or more of the following:
- DNA was not washed with the provided solutions.
Ensure the column was washed once with Wash Solution WN and twice with Wash Solution A. - Ethanol carryover.
Ensure that the dry spin under the Column Wash procedure is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
Yes, Product number 28229 is the correct replacement part for the kits tagged in "Products". It comes with 5 tubes of 1 ml each.
Yes, it is possible to isolate bacterial DNA present in tissue samples using 53100 kit. Just add 6 mg of Lysozyme (15 µL from 400 mg/mL stock solution) along with Proteinase K to the lysate during the incubation step.
Yes, it is possible to isolate DNA from bone tissue using this kit after decalcification of the tissue. Please feel free to contact our Tech support team at support@norgenbiotek.com for help with decalcification protocol.
Yes, it is possible to isolate DNA from insect samples using this kit. For large specimens, consider dissecting the insect to remove exoskeleton. For smaller specimens, grind the sample with liquid nitrogen using pestle and mortar. Please contact our Tech support team at support@norgenbiotek.com and ask for reference publications.
Magnetic Bead System
If the magnetic beads were accidently pipetted up with the supernatant, the pipette tip was placed too close to the magnetic beads while pipetting. Simply return the magnetic beads and the supernatant back into the sample well. Mix well, and place the plate back onto the magnetic separation plate for the specified time. Carefully remove the supernatant without touching the magnetic beads.
A low genomic DNA yield can be due to the following reasons:
- Incomplete lysis of cells.
Ensure that the correct lysis protocol was applied to the sample. Ensure Proteinase K was added properly. Extend the incubation time of Proteinase K digestion or reduce the amount of tissue or cells used for lysis. - Amount of magnetic beads added was not sufficient.
Ensure that the magnetic bead suspension is mixed well prior to use to avoid any inconsistency in DNA isolation. - DNA concentration in the cell or tissue sample being used is low.
Some samples contain very little target DNA. This varies from individual to individual based on numerous variables. Extending the incubation time of Proteinase K digestion or adding an incubation step at 65ºC may result in increased yields.
Lysate can be more gelatinous prior to adding the Magnetic beads and Ethanol due to the following factors:
- The lysate solution mixture is not homogeneous.
To ensure a homogeneous solution, vortex for 10-15 seconds before adding the magnetic beads to the lysate. - Maximum number of cells or amount of tissue exceeds kit specifications.
Refer to specifications to determine if the amount of starting material falls within kit specifications.
If the DNA does not perform well in downstream applications, it may be due to one or more of the following:
- DNA was not washed with 70% Ethanol.
Traces of salt from the binding step may remain in the sample if the magnetic beads are not washed with 70% Ethanol. Salt may interfere with downstream applications, and thus must be washed from the magnetic beads. - Ethanol carryover.
Ensure that the drying step after the 70% ethanol wash steps is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
RNA can get co-eluted with the DNA. Carry out a digestion with RNase A on the elution if the RNA present will interfere with downstream applications. Refer to manufacturer’s instructions regarding amount of enzyme to use, optimal incubation time and temperature.
High Throughput Magnetic Bead System
If the magnetic beads were accidently pipetted up with the supernatant, the pipette tip was placed too close to the magnetic beads while pipetting. Simply return the magnetic beads and the supernatant back into the sample well. Mix well, and place the plate back onto the magnetic separation plate for the specified time. Carefully remove the supernatant without touching the magnetic beads.
A low genomic DNA yield can be due to the following reasons:
- Incomplete lysis of cells.
Ensure that the correct lysis protocol was applied to the sample. Ensure Proteinase K was added properly. Extend the incubation time of Proteinase K digestion or reduce the amount of tissue or cells used for lysis. - Amount of magnetic beads added was not sufficient.
Ensure that the magnetic bead suspension is mixed well prior to use to avoid any inconsistency in DNA isolation. - DNA concentration in the cell or tissue sample being used is low.
Some samples contain very little target DNA. This varies from individual to individual based on numerous variables. Extending the incubation time of Proteinase K digestion or adding an incubation step at 65ºC may result in increased yields.
Lysate can be more gelatinous prior to adding the Magnetic beads and Ethanol due to the following factors:
- The lysate solution mixture is not homogeneous.
To ensure a homogeneous solution, vortex for 10-15 seconds before adding the magnetic beads to the lysate. - Maximum number of cells or amount of tissue exceeds kit specifications.
Refer to specifications to determine if the amount of starting material falls within kit specifications.
If the DNA does not perform well in downstream applications, it may be due to one or more of the following:
- DNA was not washed with 70% Ethanol.
Traces of salt from the binding step may remain in the sample if the magnetic beads are not washed with 70% Ethanol. Salt may interfere with downstream applications, and thus must be washed from the magnetic beads. - Ethanol carryover.
Ensure that the drying step after the 70% ethanol wash steps is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
RNA can get co-eluted with the DNA. Carry out a digestion with RNase A on the elution if the RNA present will interfere with downstream applications. Refer to manufacturer’s instructions regarding amount of enzyme to use, optimal incubation time and temperature.
Citations
| Title | First report of Leucinodes africensis and Leucinodes laisalis on Solanum aethiopicum and Solanum melongena in farmer's fields in southern Ghana |
| Citation | Bulletin of Entomological Research 2024. |
| Authors | Ken Okwae Fening1 , Stanley Osafo Okyere1 , Ethelyn Echep Forchibe1 , Babatoundé Ferdinand Rodolphe Layodé1 , Tegbe Enyonam Richmond1 , Lakpo Koku B. A. Agboyi2 , Kwame Afreh-Nuamah1 and Francis Onono Wamonje3 |
| Title | Structural insights into a functional unit from an immunogenic mollusk hemocyanin |
| Citation | Structure 2024. |
| Authors | Sebastián M. Muñoz Gabriel Vallejos-Baccelliere Augusto Manubens Michelle L. Salazar Andrey F.Z. Nascimento Patricio Tapia-Reyes Claudio Meneses Andre L.B. Ambrosio María Inés Becker Victoria Guixé Victor Castro-Fernandez |
| Title | Utilizing Magnetic Levitation to Detect Lung Cancer-Associated Exosomes |
| Citation | ACS Sensors 2024. |
| Authors | Alper Baran Sözmen Ahu Arslan-Yildiz* |
| Title | A Study of DNA Methylation of Bladder Cancer Biomarkers in the Urine of Patients with Neurogenic Lower Urinary Tract Dysfunction |
| Citation | Biology 2023. |
| Authors | Periklis Koukourikis, Maria Papaioannou, Petros Georgopoulos, Ioannis Apostolidis, Stavroula Pervana and Apostolos Apostolidis |
| Title | Abnormal adipogenic signaling in the bone marrow mesenchymal stem cells contributes to supportive microenvironment for leukemia development |
| Citation | Cell Communication and Signaling 2023. |
| Authors | Rawan Sabbah, Sahar Saadi, Tal Shahar-Gabay, Shiran Gerassy, Shlomit Yehudai-Resheff & Tsila Zuckerman |
| Title | Composition and Food Web Structure of Aphid-Parasitoid Populations on Plum Orchards in Chile |
| Citation | Insects 2023. |
| Authors | Jeniffer K. Alvarez-Baca , Xiomara Montealegre, Armando Alfaro-Tapia , Francisca Zepeda-Paulo, Joan Van Baaren, Blas Lavandero and Cécile Le Lann |
| Title | Diversity, Taxonomic Novelty, and Encoded Functions of Salar de Ascotán Microbiota, as Revealed by Metagenome-Assembled Genomes |
| Citation | Microorganisms 2023. |
| Authors | Veloso, Marcelo, Angie Waldisperg, Patricio Arros, Camilo Berríos-Pastén, Joaquín Acosta, Hazajem Colque, Macarena A. Varas, Miguel L. Allende, Luis H. Orellana, and Andrés E. |
| Title | Sex determination of Eastern White-crowned Sparrows (Zonotrichia leucophrys leucophrys) using wing chord length |
| Citation | Journal of Field Ornithology 2023. |
| Authors | Ryan A.C Leys and Leanne A. Grieves |
| Title | Drosophila Toll links systemic immunity to long-term intestinal epithelial integrity |
| Citation | bioRxiv 2018. |
| Authors | Atilano, M., Glittenberg, M., Hoyle, A., & Ligoxygakis, P. (2018). |
| Title | A scientific treatment approach for acute mast cell leukemia: using a strategy based on next-generation sequencing data |
| Citation | Blood Research 2016. |
| Authors | Youk, J., Koh, Y., Kim, J. W., Kim, D. Y., Park, H., Jung, W. J., ... & Lee, S |