RNA/DNA Purification Kits
For sequential isolation of total RNA and genomic DNA from the same sample
For research use only and NOT intended for in vitro diagnostics.
RNA/DNA Purification Kits
For sequential isolation of total RNA and genomic DNA from the same sample
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Sequentially isolate and purify total RNA and DNA from a single sample
- Two column system: one for DNA and one for RNA
- The RNA column is for the purification of total RNA including microRNA
- No need to split the lysate, or to use phenol or precipitation methods
- Rapid and efficient spin column procedure - it takes only 30 minutes
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
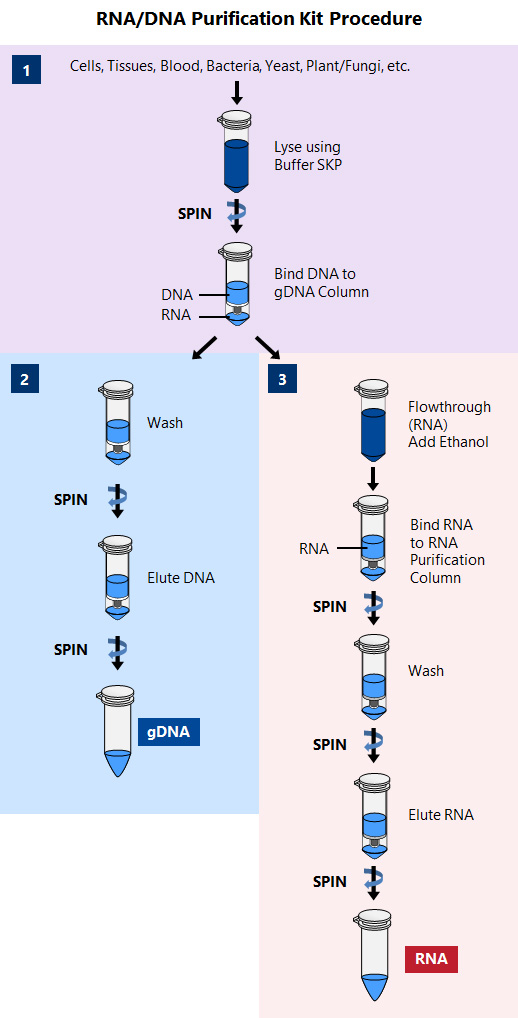
These kits provide a rapid method for the isolation and purification of total RNA and DNA sequentially from a single sample of cultured animal cells and tissues, blood, bacteria, yeast, or fungi. The lysate is passed over two columns: 1) a DNA column and 2) an RNA column. Total RNA of all sizes is purified, including microRNA. Both DNA and RNA are of the highest purity and yield.
These kits are ideal for researchers who are interested in studying the genome and transcriptome of a single sample, such as for studies of microRNA profiling, gene expression including gene silencing experiments or mRNA knockdowns, studies involving biomarker discovery, and for characterization of cultured cell lines. Norgen’s RNA/DNA Purification Kits are especially useful for researchers who are isolating macromolecules from precious, difficult to obtain or small samples such as biopsy materials or single foci from cell cultures, as they eliminate the need to fractionate the sample. Furthermore, analysis will be more reliable since the RNA and DNA are derived from the same sample, thereby eliminating inconsistent results. The purified macromolecules are of the highest purity and can be used in a number of different downstream applications
RNA/DNA Purification Kit (Spin Column)
Maximum column binding capacity of 50 μg for RNA and 20 μg for DNA.
RNA/DNA Purification Micro Kit (Micro)
The purified RNA and DNA fractions can be eluted in as little as 20 μL. Ideal for cell number inputs of 500,000 and as little as 5 cells. Maximum column binding capacity of 35 μg for RNA and 10 μg for DNA.
Details
Supporting Data
Figure 1. Recovery of True Total RNA including microRNA from Hamster Heart. Panel A is a 1X MOPS 1% agarose gel showing the RNA that was isolated from 2 different samples of 10 mg hamster heart using Norgen's RNA/DNA Purification Kit. Norgen's RNA/DNA Purification Kit isolated large RNA (represented by 28S and 18S rRNA) with high integrity. Moreover, it provided the added benefit of recovering small RNA without any additional protocol. Panel B is a result from a bioanalyzer resolution of the eluted RNA. Similar to the agarose gel, Norgen's RNA/DNA Purification Kit showed the added benefit of recovering small RNA. The effectiveness of small RNA recovery was also demonstrated by gene-specific RT-qPCR. One microgram of RNA was used in RT-qPCR reactions for human S15 (for Large RNA) and miR-21 (for microRNA) genes. The RNA isolated by Norgen’s RNA/DNA Purification Kit showed detection of both small RNA (Panel C) and the large RNA (Panel D).
Figure 2. Recovery of Intact, High Quality Genomic DNA from Hamster Heart. Panel A is a 1% agarose gel showing the gDNA isolated from the same hamster heart samples above using Norgen's RNA/DNA Purification Kit. Lane M is Norgen's HighRanger 1 kb DNA Ladder and the sample lanes contain 10 µL of each of the 100 µL elutions. The gel showed high quality, and intact genomic DNA. Panel B is the result of qPCR amplification of 25 ng of eluted genomic DNA using 5S rRNA-specific primers. Genomic DNA isolated using Norgen's RNA/DNA Purification Kit is of high quality with effective qPCR amplification.
Figure 3. Recovery of True Total RNA including microRNA from Hamster Liver. Panel A is a 1X MOPS 1% agarose gel showing 3 µL of 20 µL eluted RNA that was isolated from 2 different samples of 5 mg hamster liver using Norgen's RNA/DNA Purification Micro Kit. Norgen’s RNA/DNA Purification Micro Kit isolated large RNA (represented by 28S and 18S rRNA) with high integrity. Moreover, it provided the added benefit of recovering small RNA without any additional protocol. Panel B is a result from a bioanalyzer resolution of the eluted RNA. Similar to the agarose gel, Norgen's RNA/DNA Purification Micro Kit showed the added benefit of recovering small RNA while isolating very high quality RNA. The effectiveness in small RNA recovery was also demonstrated by gene-specific RT-qPCR. One microgram of RNA was used in RT-qPCR reactions for beta-Actin (for Large RNA) and miR-21 (for microRNA) genes. The RNA isolated by Norgen’s RNA/DNA Purification Micro Kit showed detection of both small RNA (Panel C) and the large RNA (Panel D).
Figure 4. Recovery of Intact, High Quality Genomic DNA from Hamster Liver. Panel A is a 1% agarose gel showing the gDNA isolated from the same hamster liver samples using Norgen’s RNA/DNA Purification Micro Kit. Lane M is Norgen's HighRanger 1 kb DNA Ladder and the sample lanes contain 3 µL of each of the 20 µL elutions. The gel showed high quality, and intact genomic DNA. Panel B is the result of qPCR amplification of 25 ng of eluted genomic DNA using 5S rRNA-specific primers. Genomic DNA isolated using Norgen’s RNA/DNA Purification Micro Kit is of high quality with effective qPCR amplification.
|
Kit Specifications
|
|
|
Maximum Column Binding Capacity
|
50 μg for RNA
20 μg for DNA |
|
Maximum Column Loading Volume
|
650 μL
|
|
Size of RNA Purified
|
All sizes, including small
RNA (< 200 nt) |
| Maximum Amount of Starting Material: Animal Cells Animal Tissues Blood Bacteria Yeast Fungi Plant Tissues |
5 x 106 cells 25 mg (for most tissues)** 200 μL 1 x 109 cells 1 x 108 cells 50 mg 50 mg |
| Time to Complete 10 Purifications |
30 minutes
|
|
Average Yield*:
HEK 293 Cells (1 x 106 cells) HEK 293 Cells (1 x 106 cells) Liver (15 mg) Liver (15 mg) |
10-15 μg RNA 2-4 μg DNA 30-35 μg RNA 4-6 μg DNA |
*Average Yield will vary depending upon a number of factors including species, growth conditions used, and development stage.
**Tissue inputs of up to 40 mg may be used, however for inputs greater than the recommended 25 mg, cross-contamination of the RNA and DNA fractions is possible.
Storage Conditions and Product Stability
Store Proteinase K at -20°C upon arrival. All other solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 1 year after the date of shipment.
| Component | Cat. 48700 (50 preps) | Cat. 50300 (50 preps) |
|---|---|---|
| Buffer SKP | 40 mL | 40 mL |
| Wash Solution A | 2 x 38 mL 1 x 18 mL |
2 x 38 mL 1 x 18 mL |
| Elution Solution A | 6 mL | 6 mL |
| Elution Buffer F | 15 mL | 6 mL |
| RNase-Free Water | 40 mL | 40 mL |
| Proteinase K | 2 x 12 mg | 2 x 12 mg |
| gDNA Purification Columns | 50 | - |
| gDNA Purification Micro Columns | - | 50 |
| RNA Purification Columns | 50 | - |
| RNA Purification Micro Columns | - | 50 |
| Collection Tubes | 100 | 100 |
| Elution Tubes (1.7 mL) | 100 | 100 |
| Product Insert | 1 | 1 |
Documentation
FAQs
Spin Column, Micro
Poor DNA recovery may be caused by one or more of the following:
- Incomplete lysis of cells or tissue. Ensure that the appropriate amount of Buffer SKP was used for the amount of cells or tissue.
- Column has become clogged. Do not exceed the recommended amounts of starting materials. The amount of starting material may need to be decreased if the column shows clogging below the recommended levels. See FAQ related to “Clogged Column” below.
- An alternative elution solution was used. It is recommended that the RNA Elution Buffer supplied with this kit be used for maximum RNA recovery.
- Ethanol was not added to the lysate. Ensure that the appropriate amount of ethanol is added to the lysate before binding to the column.
- Ethanol was not added to the Wash Solution A. Ensure that 96 – 100 % ethanol is added to the supplied Wash Solution prior to use.
- Low RNA content in cells or tissues used. Different tissues and cells have different RNA contents, and thus the expected yield of RNA will vary greatly from these different sources. Please check literature to determine the expected RNA content of your starting material.
- Cell Culture: Cell monolayer was not washed with PBS. Ensure that the cell monolayer is washed with the appropriate amount of PBS in order to remove residual media from cells.
- Yeast: Lyticase was not added to the Resuspension Buffer. Ensure that the appropriate amount of lyticase is added when making the Resuspension Buffer.
- Bacteria and Yeast: All traces of media not removed. Ensure that all media is removed prior to the addition of the Buffer SKP through aspiration.
Column clogging may occur due to:
- Maximum number of cells or amount of tissue exceeds kit specifications. Refer to specifications to determine if the amount of starting material falls within kit specifications.
- Centrifuge temperature is too low. Ensure that the centrifuge remains at room temperature throughout the procedure. Temperatures below 20°C may cause precipitates to form that can cause the columns to clog.
Eluted RNA can be degraded due to the following factors:
- RNase contamination. RNases may be introduced during the use of the kit. Ensure proper procedures are followed when working with RNA. Please refer to “Working with RNA” at the beginning of this user guide.
- Procedure not performed quickly enough. To maintain the integrity of the RNA, it is important that the procedure be performed quickly. This is especially important for the Cell Lysate Preparation Step in the Animal Tissue protocol, as RNA in animal tissues is not protected after harvesting until it is disrupted and homogenized. Also, after the DNA binding step, the flowthrough should be kept on ice or –20°C if the RNA purification step is not carried out immediately.
- Improper storage of the purified RNA. For short-term storage, RNA samples may be stored at –20°C for a few days. It is recommended that samples be stored at –70°C for longer-term storage.
- Frozen tissues or cell pellets were allowed to thaw prior to RNA isolation. Do not allow frozen tissues to thaw prior to grinding with the mortar and pestle to ensure that the integrity of the RNA is not compromised.
- Lysozyme or lyticase used may not be RNase-free. Ensure that the lysozyme and lyticase being used with this kit are RNase-free to prevent possible problems with RNA degradation.
- Tissue samples were frozen improperly. Samples should be flash-frozen in liquid nitrogen and transferred immediately to a –70°C freezer for long-term storage.
If the RNA does not perform well in downstream applications, it may be due to one or more of the following:
- RNA was not washed twice with the provided Wash Solution A. Traces of salt from the binding step may remain in the sample if the column is not washed twice with Wash Solution A. Salt may interfere with downstream applications, and thus must be washed from the column.
- Ethanol carryover. Ensure that the dry spin under the RNA Wash procedure is performed to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
Citations
| Title | Noise Stress Abrogates Structure-Specific Endonucleases within the Mammalian Inner Ear |
| Citation | International Journal of Molecular Sceinces 2024. |
| Authors | O'neil W. Guthrie |
| Title | Filarial nematodes in domestic dogs and mosquitoes (Diptera: Culicidae) from semi-rural areas in Central Chile |
| Citation | Frontiers in Veterinary Science 2024. |
| Authors | Beatriz Cancino-Faure1* Christian R. González2 Alejandro Piñeiro González1 Soledad Pinochet3 Sofía Bustos1 Rodrigo Morchón4 Alejandro Piñeiro Cazaux5 Ivonne Quezada Aguilar5 Merayot Salas Espinoza5 Rodrigo Acevedo Salgado5 Carmen Barra Díaz5 Christian Segovia1,6 Rafael Lozada-Yavina1,7 Cristian A. Álvarez Rojas8* |
| Title | Gene expression profiling of endomyocardial biopsies from dogs with dilated cardiomyopathy phenotype |
| Citation | Veterinary Cardiology 2024. |
| Authors | Antonio Di Loria, Carlo Ferravante, Ylenia D'Agostino, Giorgio Giurato, Massimiliano Tursi, Elena Grego, Manuela Perego, Alessandro Weisz, Paolo Ciaramella, Roberto Santilli |
| Title | Host genetic and immune factors drive evasion of HIV-1 pathogenesis in viremic non-progressors |
| Citation | Cell Press 2024. |
| Authors | Ángel Bayón-Gil · Inmaculada Hernández · Judith Dalmau · Juan C. Nieto · Víctor Urrea · Lidia Garrido-Sanz · Ginevra Caratú · Maria C. García-Guerrero · Cristina Gálvez · María Salgado · Itziar Erkizia · Fernando Laguía · Patricia Resa-Infante · Marta Massanella · Raúl Tonda · Jordi Morata · Kai Ying Hong · Jane Koshy · Aaron R. Goldman · Leila Giron · Mohamed Abdel-Mohsen · Holger Heyn · Javier Martinez-Picado · Maria C. Puertas |
| Title | LncRNA regulation in human oocytes obtained from patients with polycystic ovaries |
| Citation | ACADEMIC JOURNAL OF HEALTH SCIENCES 2024. |
| Authors | Warda Rai1 , Hakan Aytaçoglu1 , Burcu Özbakir2,3 , Cenk Serhan Özverel3,4 , Emine Kandemis1 , Pinar Tulay |
| Title | Novel enhancer mediates the RPL36A-HNRNPH2 readthrough loci and GLA gene expressions associated with fabry disease |
| Citation | Frontiers in Genetics 2023. |
| Authors | Mohammed A. Al-Obaide, Saimul Islam, Ibtisam Al-Obaidi, and Tetyana L. Vasylyeva |
| Title | High aspect ratio nanomaterial-induced macrophage polarization is mediated by changes in miRNA levels |
| Citation | Frontiers in Immunology 2023. |
| Authors | Johanna Samulin Erdem, Tána Závodná, Torunn K. Ervik, Øivind Skare,Tomáš Hron, Kristine H. Anmarkrud, Anna Kusnierczyk, Julia Catalán, Dag G. Ellingsen, Jan Topinka, Shan Zienolddiny-Narui |
| Title | Multi-omics approach reveals dysregulated genes during hESCs neuronal differentiation exposure to paracetamol |
| Citation | Iscience 2023. |
| Authors | Mari Spildrejorde, Athina Samara Ankush Sharma, Magnus Leithaug, Martin Falck, Stefania Modafferi, Arvind Y.M. Sundaram, Ganesh Acharya, Hedvig Nordeng, Ragnhild Eskeland, Kristina Gervin, and Robert Lyle |
| Title | Mediation of the Same Epigenetic and Transcriptional Effect by Independent Osteoarthritis Risk-Conferring Alleles on a Shared Target Gene, COLGALT2 |
| Citation | Arthritis & Rheumatology 2023. |
| Authors | Yulia S. Kehayova, J. Mark Wilkinson, Sarah J. Rice, John Loughlin |
| Title | Optimal culture conditions are critical for efficient expansion of human testicular somatic and germ cells in vitro |
| Citation | Fertility and Sterility 2017. |
| Authors | Gat, I., Maghen, L., Filice, M., Wyse, B., Zohni, K., Jarvi, K., ... & Librach, C |