Saliva DNA Collection and Preservation Devices (50)
For simple and non-invasive saliva collection and preservation of DNA in saliva samples at ambient temperature


For research use only and NOT intended for in vitro diagnostics.
CE-IVDR marked diagnostic version available here
Saliva DNA Collection and Preservation Devices (50)
For simple and non-invasive saliva collection and preservation of DNA in saliva samples at ambient temperature
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Reliable and cost-effective
- Non-invasive, user-friendly sample collection and preservation in one convenient kit
- Preserved DNA is stable for 2 years at ambient temperature
- Compatible with any commercial DNA isolation methods including Norgen’s Saliva DNA Isolation Kits (Cat. RU45400)
- Samples are non-infectious and can be handled and shipped safely
- Shipping accessories can be purchased separately
- High quality DNA is suitable for sensitive downstream applications including PCR, qPCR, sequencing, SNP analysis, microarrays, RFLP and Southern Blot Analysis
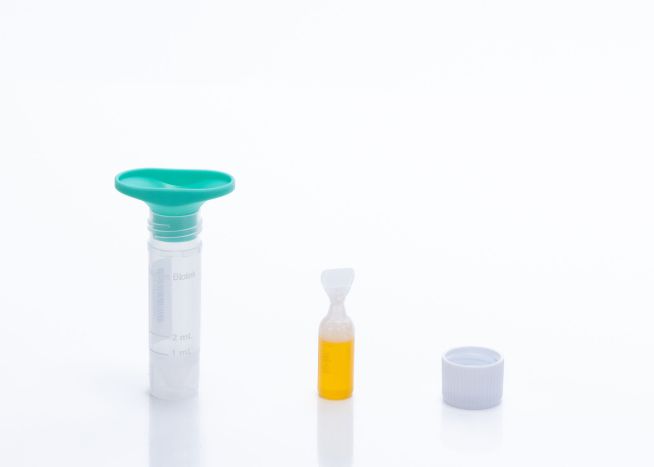
Norgen's Saliva DNA Collection and Preservation Devices are designed for 1) simple and non-invasive saliva collection and 2) preservation of DNA in saliva samples at ambient temperature. Each of the 50 Saliva DNA Collection and Preservation Devices consists of 3 components:
- Saliva Collection Funnel and Collection Tube
- Collection Tube Cap
- Norgen's Saliva DNA Preservative contained within a sealed squeezable ampoule
Saliva samples are collected by spitting inside the Collection Funnel which has been assembled with the Collection Tube. After collecting the required volume of saliva the Collection Funnel is removed and the contents of the Preservative Ampoule are then added and mixed with the collected saliva. The Saliva Collection Tube is subsequently sent to the laboratory for DNA isolation and analysis. DNA can be isolated from the preserved saliva samples using Norgen's Saliva DNA Isolation Kit (Cat. RU45400). Each of Norgen's Collection Tubes is labeled with a unique serial number that can be used for secure and anonymous tracking of the sample. The saliva DNA in preserved samples is stable for more than 2 years at room temperature. This kit is ideal for collecting and preserving DNA samples for epidemiological and population studies.
Saliva DNA Preservative
Norgen's Saliva DNA Preservative is an aqueous storage buffer designed for rapid cellular lysis and subsequent preservation of DNA from fresh specimens. The buffer prevents the growth of Gram-negative and Gram-positive bacteria and fungi, and also inactivates viruses allowing the resulting non-infectious samples to be handled and shipped safely. In addition, the buffer eliminates the need to immediately process or freeze samples and allows the samples to be shipped to centralized testing facilities at ambient temperature. The components of the buffer allow samples to be stored for more than 2 years without any detectable DNA degradation.
DNA Isolation from Preservative
Prior to saliva DNA isolation, vortex the Collection Tube containing preserved saliva for 10 seconds and incubate at 55°C for one hour. Saliva DNA can now be isolated from the preserved saliva samples using any commercially available method, including Norgen’s Saliva DNA Isolation Kit (Cat. RU45400), Norgen’s Saliva DNA Isolation 96-Well Kit (Cat. RU35200) and Norgen’s Saliva DNA Isolation Kit (Magnetic Bead System) (Cat. RU55400, RU62900). Also, Norgen’s Saliva DNA Isolation Reagent Kit (up to 4 mL) (Cat. RU35720) allows for DNA isolation from the preserved saliva samples using an alcohol precipitation method. The purified DNA is of the highest quality and can be used in a number of downstream applications including PCR, NGS and microarray analysis.
Details
Supporting Data
Figure 1. Stability of DNA Preserved in Norgen's Saliva DNA Preservative at Room Temperature for over 2 Years. Saliva samples were collected from numerous donors and mixed, and then an equal volume of Norgen's Saliva DNA Preservative was added to the saliva. The preserved saliva was then stored at room temperature for up to 24 months. Saliva DNA was subsequently isolated at 4 months, 8 months, 16 months and 24 months from 0.5 mL of the saliva/preservative sample using Norgen's Saliva DNA Isolation Kit (Cat. RU45400). For visual analysis, 100 µL of the purified DNA was run on an agarose TAE gel. As it can be seen, there is no evidence of DNA degradation after the saliva samples are stored for 24 months at ambient temperatures in Norgen's Saliva DNA Preservative. Furthermore, the size of the DNA was maintained over 24 kb for the entire 24 month period. M: Norgen's UltraRanger 1 Kb DNA Ladder (Cat. 12100).
Figure 2. GAPDH Real Time PCR Amplification of Saliva DNA from Saliva Samples Stored at Room Temperature from Day 0 up to 24 Months. All isolated saliva DNA samples were successfully amplified without any sign of PCR inhibition and difference in Ct. Red: Day 0, Blue: 4 months, Purple: 8 months, Green: 16 months and Sky blue: 24 months. Black: No template control.
Figure 3. V3-V4 16s rRNA PCR amplification for Illumina MiSeq 16s rRNA library preparation. The saliva DNA was isolated using Norgen's Saliva DNA Isolation Kit (Cat. RU45400) from saliva that had been preserved for various periods of time up to 2 years at room temperature in Norgen’s Saliva DNA Preservative. The PCR result shows the clean PCR amplification at ~550 bp indicating the right target of V3-V4 16s rRNA , with no difference between day 0 and 24 months. M: FastRunner DNA Ladder (Cat. 12800).
|
Kit Specifications
|
|
|
Volume of Saliva Collected
|
2 mL
|
|
Volume of Saliva-Preservative Mix
|
4 mL
|
|
Preservation Temperature
|
Room Temperature
|
| Preservation time |
Over 2 years at room temperature
|
Shelf Life and Handling
- When stored at room temperature, unused Norgen Saliva Preservative Tubes are stable through to the collect-before date without any reduction in performance. Please see the device insert or tube label for collect-before date.
- Once collected, saliva is stable for more than 2 years when kept tightly sealed and stored at room temperature.
- The Collection Tube, the Collection Funnel and the Device Container of each Individual Saliva DNA Collection and Preservation Device are recyclable.
| Kit Components | Cat. RU49000 (50 Devices) |
|---|---|
| Individual Saliva DNA Collection and Preservation Devices | 50 |
| Donor Procedure Flowchart | 1 |
| Product Insert | 1 |
| Individual Saliva DNA Collection and Preservation Device Contents | |
| Saliva Collection Funnel and Collection Tube | 1 |
| Collection Tube Cap | 1 |
| Preservative Ampoule | 1 |
| Donor Instructions | 1 |
Documentation
Inhibitory Effect of Norgen’s Saliva DNA Preservative on the Growth of Bacteria and Yeast
Isolation of High Quality Saliva DNA from Variable Donor Saliva Samples
Long Term Stability of DNA Stored in Norgen’s Saliva DNA Preservative
The Range of DNA Yield with Norgen’s Saliva DNA Collection, Preservation and Isolation Kit
Sequencing Analysis of Saliva DNA Isolated using Norgen’s Saliva DNA Collection, Preservation and Isolation Kit
A Comparative Study Between Two Saliva DNA Preservation Systems, and Two Column-Based Saliva DNA Purification Methods
Comparison of Norgen’s Saliva DNA Collection, Preservation and Isolation Kit to a Leading Competitor’s Kit
DNA Isolation from Saliva Preserved using Norgen’s Saliva DNA Collection and Preservation Device using Beckman Coulter Agencourt
DNA Isolation from Saliva Preserved with Norgen’s Saliva DNA Collection and Preservation Device using Qiagen’s QIAamp DNA Blood
Stability of DNA Stored in Norgen’s Saliva DNA Preservative for 52 Months at Room Temperature
The Use of Saliva Swabs in Norgen’s Saliva DNA Collection, Preservation and Isolation Kit
Linearity of DNA Isolated from Increasing Volumes of Preserved Saliva Using Norgen’s Saliva DNA Isolation Kit
The Effect of Elution Volume on DNA Quantity and Quality Using Norgen’s Saliva DNA Isolation Kit
Comparison of DNA Isolated from Saliva using Norgen’s Preservative and Saliva DNA Isolation Kit Vs Competitor's
Stability of DNA Stored in Norgen’s Saliva DNA Preservative at 55°C
Efficient Restriction Enzyme Digestion of Saliva DNA isolated using Norgen’s Saliva DNA Collection, Preservation & Isolation Kit
Rapid and qPCR ready DNA isolation from environmental and human bodily fluid samples using Magnetic bead system
Viability Of Microorganisms in Saliva & Stool Collection and Transportation Preservatives
FAQs
Each saliva tube is a 5 mL tube with dimensions as follows: 65 mm (height) x 16.5 mm (diameter).
Tube material is as follows:
- Cap: Polyethylene
- Tube: Polypropylene (PP)
- Funnel: Polyphenylene ether Polystyrene (PPE-PS) thermoplastic
Citations
| Title | At age 9, the methylome of assisted reproductive technology children that underwent embryo culture in different media is not significantly different on a genome-wide scale |
| Journal | Human Reproduction. 2022. |
| Authors | Rebekka M Koeck, Florence Busato, Jorg Tost, Heleen Zandstra, Sylvie Remy, Sabine Langie, Marij Gielen, Ron van Golde, John C M Dumoulin, Han Brunner, Masoud Zamani Esteki, Aafke P A van Montfoort |
| Title | Exposure to traffic-related air pollution and bacterial diversity in the lower respiratory tract of children |
| Journal | PLOS ONE. 2021 |
| Authors | Niemeier-Walsh, C., Ryan, P. H., Meller, J., Ollberding, N. J., Adhikari, A., & Reponen, T. |
| Title | Exploring influences on food choice in a large population sample: the Italian Taste Project |
| Journal | Food Quality and Preference. 2017 |
| Authors | E. Monteleone, S. Spinelli, C. Dinnella, I. Endrizzi, M. Laureati, E. Pagliarini, F. Sinesio, F. Gasperi, L. Torri, E. Aprea, L.I. Bailetti, A. Bendini, A. Braghieri, C. Cattaneo, D. Cliceri, N. Condelli, M.C. Cravero, A. Del Caro, R. Di Monaco, S. Drago, S. Favotto, R. Fusi, L. Galassi, T. Gallina Toschi, A. Garavaldi, P. Gasparini, E. Gatti, C. Masi, A. Mazzaglia, E. Moneta, E. Piasentier, M. Piochi, N. Pirastu, S. Predieri, A. Robino, F. Russo, F. Tesini |
| Title | Sugar-Sweetened Beverage Consumption Is Adversely Associated with Childhood Attention Deficit/Hyperactivity Disorder |
| Journal | International Journal of Environmental Research and Public Health. 2016. |
| Authors | Yu CJ, Du JC, Chiou HC, Feng CC, Chung MY, Yang W, Chen YS, Chien LC, Hwang B, Chen ML |
| Title | Detection of Novel Integrons in the Metagenome of Human Saliva |
| Journal | PLOS ONE. 2016. |
| Authors | Tansirichaiya S, Rahman MA, Antepowicz A, Mullany P, Roberts AP |
| Title | Increased risk of attention-deficit/hyperactivity disorder associated with exposure to organophosphate pesticide in Taiwanese children |
| Journal | Andrology. 2016. |
| Authors | Yu CJ, Du JC, Chiou HC, Chung MY, Yang W, Chen YS, Fuh MR, Chien LC, Hwang B, Chen ML |
| Title | Attention Deficit/Hyperactivity Disorder and Urinary Nonylphenol Levels: A Case-Control Study in Taiwanese Children |
| Journal | PLOS ONE. 2016. |
| Authors | Yu CJ, Du JC, Chiou HC, Yang SH, Liao KW, Yang W, Chung MY, Chien LC, Hwang B, Chen ML |
| Title | Functional polymorphisms in the P2X7 receptor gene are associated with stress fracture injury |
| Journal | Purinergic Signalling. 2016. |
| Authors | Varley I, Greeves JP, Sale C, Friedman E, Moran DS, Yanovich R, Wilson PJ, Gartland A, Hughes DC, Stellingwerff T, Ranson C, Fraser WD, Gallagher JA |
| Title | Selective familiarity deficits in otherwise cognitively intact aging individuals with genetic risk for Alzheimer's disease |
| Journal | Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2015. |
| Authors | Dorothee Schoemaker, Judes Poirier, Sophia Escobar, Serge Gauthier, Jens Pruessner |
| Title | The dentin phosphoprotein repeat region and inherited defects of dentin |
| Journal | Molecular Genetics & Genomic Medicine. 2015. |
| Authors | Jie Yang, Kazuhiko Kawasaki, Moses Lee, Bryan M. Reid, Stephanie M. Nunez, Murim Choi, Figen Seymen, Mine Koruyucu, Yelda Kasimoglu, Ninna Estrella-Yuson, Brent P. J. Lin, James P. Simmer and Jan C.-C. Hu |
| Title | Taurodontism, variations in tooth number, and misshapened crowns in Wnt10a null mice and human kindreds. |
| Journal | Molecular Genetics & Genomic Medicine. 2014. |
| Authors | Jie Yang, Shih-Kai Wang, Murim Choi, Bryan M. Reid, Yuanyuan Hu, Yuan-Ling Lee, Curtis R. Herzog, Hera Kim-Berman, Moses Lee, Paul J. Benke, K. C. Kent Lloyd, James P. Simmer and Jan C.-C. Hu. |
| Title | Patterns of Admixture and Population Structure in Native Populations of Northwest North America. |
| Journal | PLOS Genetics. 2014. |
| Authors | Paul Verdu, Trevor J. Pemberton, Romain Laurent, Brian M. Kemp, Angelica Gonzalez-Oliver, Clara Gorodezky, Cris E. Hughes, Milena R. Shattuck,Barbara Petzelt, Joycelynn Mitchell, Harold Harry, Theresa William, Rosita Worl, Jerome S. Cybulski, Noah A. Rosenberg, Ripan S. Malhi. |
| Title | Association of premenstrual/menstrual symptoms with perinatal depression and a polymorphic repeat in the polyglutamine tract of the retinoic acid induced 1 gene |
| Journal | Journal of Affective Disorders. 2014. |
| Authors | Ene-Choo Tan, Hui-San Tan, Tze-Ern Chua, Theresa Lee, Jasmine Ng, Ying-Chia Ch’ng, Chih-Huei Choo, Helen Y Chen |
| Title | RANK/RANKL/OPG pathway: Genetic associations with stress fracture period prevalence in elite athletes. |
| Journal | The Bone Journal. 2014. |
| Authors | Ian Varley, David C. Hughes, Julie P. Greeves, Trent Stellingwerff, Craig Ranson, William D. Fraser, Craig Sale. |
| Title | Differences in the quantity of DNA found in the urine and saliva of smokers versus nonsmokers: implications for the timing of epigenetic events. |
| Journal | Epigenomics. 2012. |
| Authors | Simkin M, Abdalla M, El-Mogy M, Haj-Ahmad Y. |