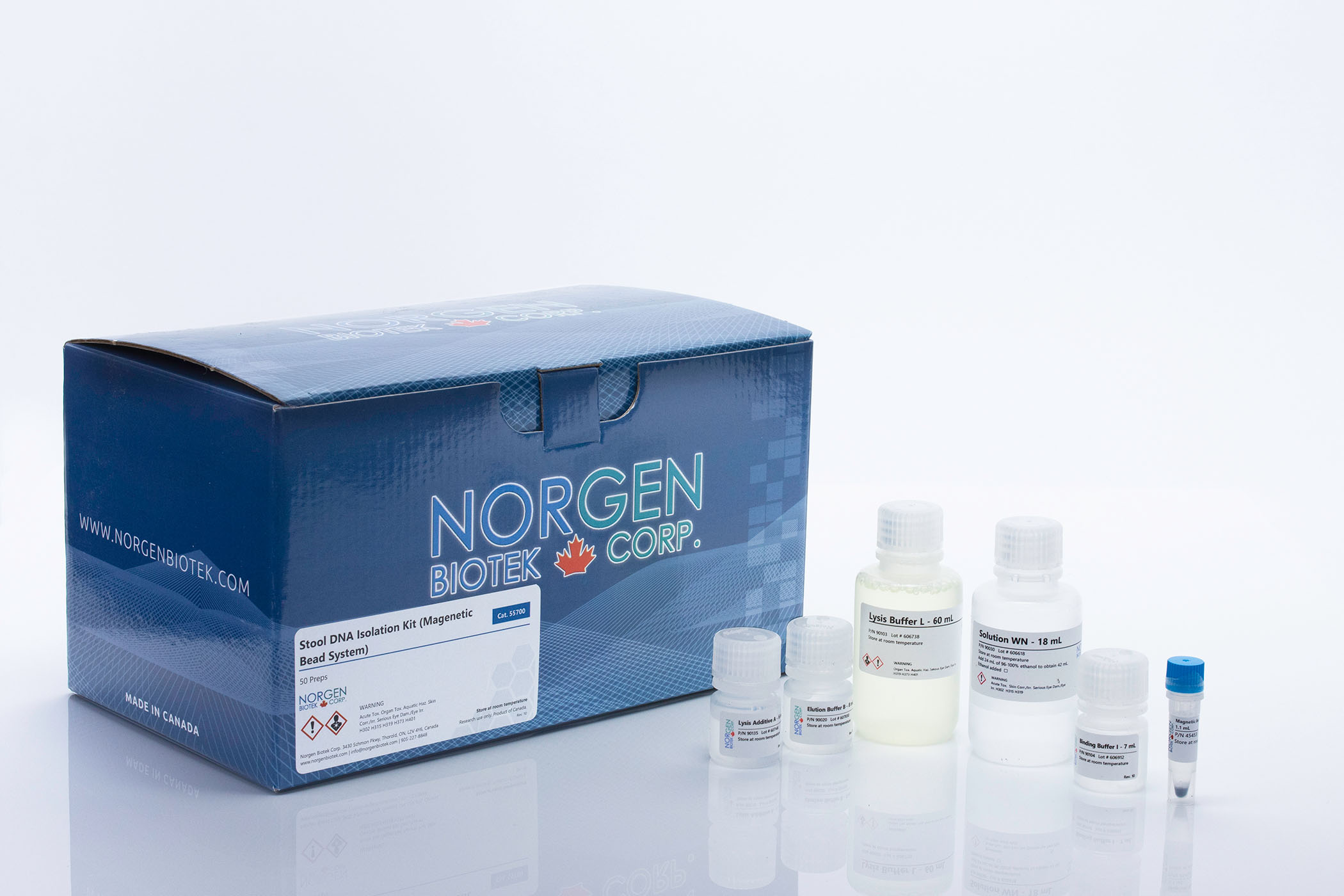
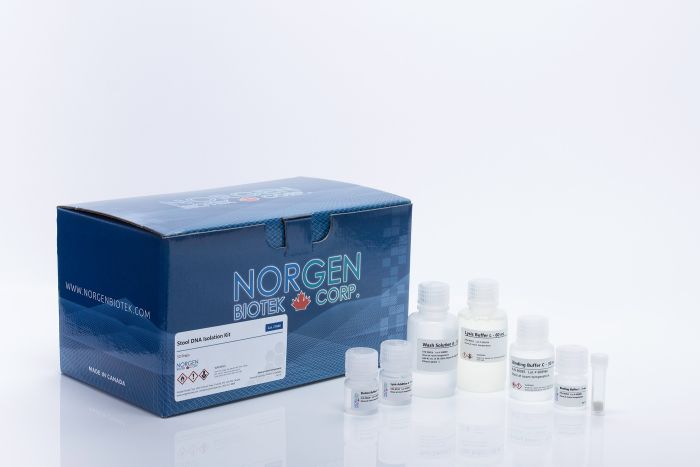
Stool DNA Isolation Kits
For the rapid and simple purification of bacterial and host DNA from stool and fecal samples.

For research use only and NOT intended for in vitro diagnostics.
CE-IVDR marked diagnostic version available here
Stool DNA Isolation Kits
For the rapid and simple purification of bacterial and host DNA from stool and fecal samples.
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
-
Simultaneous Isolation:
Host and microbial DNA using a universal protocol.
-
Compatibility:
Works with fresh, frozen, or preserved stool samples. Check out Norgen's Stool Nucleic Acid Collection and Transport Tubes
-
High-Quality DNA:
Get HMW DNA up to 50 kb and above. Suitable for PCR, qPCR, sequencing, and microarray applications.
-
Eliminates PCR Inhibitors:
Removes humic acids and other inhibitors.
-
Lysis Efficiency:
Combines chemical and physical homogenization for efficient sample lysis.
-
Available in multiple formats to suit your choice of workflow
(See below table).
| Available formats | |||||
|---|---|---|---|---|---|
| SKU | Format | Size | Preps | Input Volume | Special Features |
| 27600 | Spin Column | Mini | 50 | 200 mg or 400 µL | User friendly |
| 65600 | High Throughput | Mini | 192 | 200 mg or 400 µL | 96 well plate format |
| 55700 | Magnetic Bead System | Mini | 50 | 200 mg or 200 µL | Yields more HMW DNA |
| 63100 | High Throughput Magnetic Bead System | Mini | 192 | 200 mg or 200 µL | Automation friendly |

Free Download
Extracting Biological Insights from Stool
Tips and tricks for isolating high yield and quality DNA, RNA, miRNA and EV's from fecal samples
Download for FreeDetails
Supporting Data
Figure 1. Higher Yields of DNA than Competitor Q. Stool DNA was isolated from 200 mg of fresh or preserved stool samples using Norgen’s Stool DNA Isolation Kit and Competitor Q's Kit. For evaluation, 10 µL of DNA from the elution was run on 1X TAE 1.2% agarose gel. Norgen's kit isolated much higher yields of DNA. *Stool was collected using Norgen’s Stool Nucleic Acid Collection and Preservation tubes (Cat. 45660). Marker = Norgen’s HighRanger DNA Ladder (Cat. 11900).
Figure 2. Stool DNA quality and concentration measured by NanoDrop. High DNA concentration and quality was obtained using Norgen’s Stool DNA Isolation Kit from fresh or preserved stool samples. Figure 2A: Fresh stool samples, Figure 2B: Preserved stool using Norgen’s Stool Nucleic Acid Collection and Preservation Tubes (Cat. 45660).
Figure 3. Detection of 16S rRNA from stool DNA isolated from 200 mg of fresh stool samples using Norgen's Stool DNA Isolation Kit and Competitor Q’s kit. DNA quality was confirmed by Real-time PCR using 2 µL of stool DNA (total PCR reaction volume was 20 µL) to detect 16S rRNA from different microorganisms in the stool samples. The earlier Ct value with Norgen's DNA samples (blue lines) compared to Competitor Q’s samples (red lines) indicated a higher quality of stool DNA for downstream applications. Figure 3A: Fresh stool sample, Figure 3B: Preserved stool using Norgen’s Stool Nucleic Acid Collection and Preservation Tubes (Cat. 45660).
Figure 4. Detection of 5S rRNA from the Stool DNA isolated from 200 mg of fresh stool samples using Norgen's Stool DNA Isolation Kit and Competitor Q’s Kit. DNA quality was confirmed by Real-time PCR using 2 µL of stool DNA (total PCR reaction volume was 20 µL) to detect 5S rRNA from eukaryotic DNA in the stool samples. The earlier Ct value with Norgen's DNA samples (blue lines) compared to Competitor Q’s samples (red lines) indicated a higher quality of stool DNA for downstream applications. Figure 4A: Fresh stool sample, Figure 4B: Preserved stool using Norgen’s Stool Nucleic Acid Collection and Preservation Tubes (Cat. 45660).
Figure 5. Better 16S rRNA detection from DNA isolated using Norgen’s Stool DNA Preservative and Stool DNA Isolation Kit. Two microlitres of stool DNA was used in a 20 µL PCR reaction volume. Stool was preserved using Norgen’s Stool DNA Preservative and isolated using Norgen’s Stool DNA Isolation Kit showed better 16S rRNA gene detection as compared to fresh stool isolated with Competitor Q.
Figure 6. Distribution of 10 Different Fecal Microbiomes. A) Principal Coordinate Analysis of 10 fecal microbiomes showing differences in the distribution of taxonomic classifications between samples up to class level. B) Hierarchical clustering of 10 fecal microbiomes based on genus-level classifications, including a bar chart showing the relative abundance of genus-level classifications for each sample in the dendrogram.
Figure 7. High Quality DNA Isolated from Preserved Stool Samples. DNA was isolated from 200 µL preserved stool samples using Norgen’s Stool DNA Isolation Kit (Magnetic Bead System).For evaluation, 10 µL from 75 µL of elution were run on 1X TAE 1.2% agarose gel. Stool samples were preserved in Norgen’s Stool Nucleic Acid Collection and Transport Tubes (Cat. 45630, 45660). M = Norgen’s HighRanger DNA Ladder (Cat. 11900).
Figure 8. Comparison of A260/280 and A260/230 ratios. DNA was isolated from 200 mg stool samples using Norgen's Stool DNA Isolation Kit (Magnetic Bead System) and Norgen's Stool DNA Isolation Kit (column format). The purified DNA was then compared for A260/280 and A260/230 ratios. DNA isolated using Norgen’s Stool DNA Isolation Kit (Magnetic Bead System) (A, B and C) showed a comparable DNA quality to the DNA isolated using Norgen’s Stool DNA Isolation (Column method), indicating the consistence and highest DNA quality were generated by the Stool DNA Isolation Kit (Magnetic Bead System).
Figure 9. High Quality DNA confirmed by Real-time PCR. DNA was isolated from 200 mg stool using Norgen's Stool DNA Isolation Kit (Magnetic Bead System) and Norgen's Stool DNA Isolation Kit (column format). The quality of the purified DNA was evaluated by using 8 μL of stool DNA (total PCR reaction volume was 20 µL) to detect 16S rRNA from the different stool samples. No PCR inhibition was observed from DNA isolated using Norgen's Stool DNA Isolation Kit (Magnetic Bead System) (blue circles), similar to the DNA isolated using Norgen’s Stool DNA Isolation Kit (Column method) (red circles), indicating the excellent isolation consistency and the quality of the stool DNA for downstream applications.
Figure 10. 16S Metagenomics data generated by Illumina MiSeq. Stool DNA was isolated using Norgen’s Stool DNA Isolation Kit (Magnetic Bead System) from 200 mg of stool from healthy donors, and the stool microbiomes were successfully sequenced by Illumina MiSeq.
Figure 11. High Quality DNA Isolated from Preserved Stool Samples. DNA was isolated from 200 µL preserved stool samples using Norgen’s Stool DNA Isolation 96-Well Kit (Magnetic Bead System). For evaluation, 10 µL from 75 µL of elution were run on 1X TAE 1.2% agarose gel. M = Norgen’s HighRanger DNA Ladder (Cat. 11900).
Figure 12. High Quality DNA Confirmed by Real-Time PCR. DNA was isolated from 200 mg stool using Norgen's Stool DNA Isolation 96-Well Kit (Magnetic Bead System). The quality of the purified DNA was evaluated by using 2 µL of stool DNA (total PCR reaction volume was 20 µL) to detect 16S rRNA from the stool sample. No PCR inhibition was observed from DNA isolated using Norgen's Stool DNA Isolation 96-Well Kit (Magnetic Bead System) indicating the excellent isolation consistency and the high quality of the stool DNA for downstream applications.
|
Kit Specifications
|
|
|
Maximum Stool Input
|
200 mg (fresh/frozen stool) or 400 μL (preserved stool)
|
|
Type of Stool Processed
|
Frozen, fresh or preserved stool
|
| Format | Spin Column |
|
Maximum Column Binding Capacity
|
50 μg
|
| Maximum Column Loading Volume |
650 μL
|
| Elution Volume | 50 μL |
| Time to Complete 10 Purifications |
30 minutes
|
| Applications |
PCR, qPCR, Southern Blot Analysis, |
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 2 years after the date of shipment.
| Component | Cat. 27600 (50 preps) | Cat. 65600 (192 preps) | Cat. 55700 (50 preps) | Cat. 63100 (192 preps) |
|---|---|---|---|---|
| Lysis Buffer L | 60 mL | 2 x 105 mL | 60 mL | 3 x 60 mL 1 x 30 mL |
| Lysis Additive A | 6 mL | 25 mL | 6 mL | 25 mL |
| Binding Buffer I | 7 mL | 25 mL | 7 mL | 25 mL |
| Binding Buffer C | 30 mL | 110 mL | - | - |
| Wash Solution A | 18 mL | 2 x 38 mL | - | - |
| Magnetic Bead Suspension | - | - | 1.1 mL | 4 x 1.1 mL |
| Solution WN | - | - | 18 mL | 55 mL |
| Elution Buffer B | 8 mL | 30 mL | 8 mL | 30 mL |
| Bead Tubes | 50 | 200 | 50 | 196 |
| Mini Spin Columns | 50 | - | - | - |
| 96-Well Plate | - | 2 | - | 2 |
| Adhesive Tape | - | 4 | - | 2 |
| Collection Tubes | 50 | - | - | - |
| 96-Well Collection Plate | - | 2 | - | - |
| Elution Tubes (1.7 mL) | 50 | - | 50 | - |
| 96-Well Elution Plate | - | 2 | - | 2 |
| Product Insert | 1 | 1 | 1 | 1 |
Automation
Stool DNA Automated Isolation Tutorial
Supplementary Protocol
Supplementary Protocol - Automated Procedure for Stool DNA Isolation Kit (Magnetic Bead System)
Documentation
(65600) Stool DNA Isolation 96-Well Kit (High Throughput) - Protocol (2x96-Well)
(55700) Stool DNA Isolation Kit (Magnetic Bead System) - Protocol (50 prep)
(63100) Stool DNA Isolation 96-Well Kit (High Throughput Magnetic Bead System) - Protocol (2 x 96-well)
Detection of Viral, Bacterial and Human Genomic DNA from Preserved Stool Samples
The Isolation of High Quality Stool DNA and its Application to Quantitative Adenovirus Detection using TaqMan® Real-Time PCR
Comparison of Stool DNA Isolation Methods for Bacterial and Mammalian DNA Detection
Determination of the DNA Molecular Weight (MW) from different Norgen Columns and Isolation Methods
Immediate Viral Inactivation Using Norgen’s Stool Nucleic Acid Preservative
Gut Microbiome Diversity: Comparison of Stool DNA Preservation Methods
Comparative Microbiome Profiles from Fecal Preservation Methods
Automation of Stool DNA Isolation on the Hamilton Vantage Using Norgen’s Magnetic Bead Stool Isolation Kit
FAQs
Spin Column
Poor DNA recovery could be due to one or more of the following:
- Homogenization was incomplete.
Depending on the type of stool, further vortexing with the flat bed vortex or bead beater equipment may be required. However, it is not recommended to increase the vortex time to longer than 5 minutes at maximum speed. Also, ensure that the maximum input of 200 mg of stool is not exceeded, as this may also cause incomplete homogenization. - An alternative elution buffer was used.
It is recommended that the Elution Buffer B supplied with this kit be used for maximum DNA recovery. - Lysis Additive A was not added to the lysate.
Ensure that the provided Lysis Additive A is added to separate humic acid and increase DNA yield. Also, an incubation can be performed at 65°C for 10 minutes after addition of the Lysis Additive A and prior to vortexing to maximize DNA recovery. - Ethanol was not added to the lysate.
Ensure that an equal amount of ethanol is added to the lysate before binding to the column. - Ethanol was not added to the Wash Solution A.
Ensure that 42 mL of 96 - 100% ethanol is added to the supplied Wash Solution A prior to use.
If the DNA does not perform well in downstream applications, It may be due to one or more of the following:
- Eluted DNA sample is brown.
Ensure that the Lysis Additive A is added. Also ensure Binding Solution I is added to the lysate and that it is incubated on ice for 10 minutes prior to spinning down the lysate. Avoid any contact with the pellet or surface residue when collecting the supernatant after the 5 minute spin during sample preparation. - Lysis Additive A was not added to the lysate.
Ensure that the provided Lysis Additive A is added to the lysate. - DNA was not washed with the provided Binding Buffer C and Wash Solution A.
Traces of salt from the binding step may remain in the sample if the column is not washed three times with the provided Binding Buffer C and Wash Solution A. Salt may interfere with downstream applications, and thus must be washed from the column. - Ethanol carryover.
Ensure that the dry spin under the "Column Wash" procedure is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications. - Binding Buffer I was not added to the lysate.
Ensure that the Binding Buffer I is added to the lysate and that it is incubated on ice for 10 minutes prior to spinning down the lysate. - PCR reaction conditions need to be optimized.
Take steps to optimize the PCR conditions being used, including varying the amount of template, changing the source of Taq polymerase, looking into the primer design and adjusting the annealing conditions.
Yes, the Stool DNA Isolation Kit is capable of simultaneous extraction of both host DNA and microbial DNA (universal protocol). Please contact our technical support team at support@norgenbiotek.com and ask for reference publications.
Our Stool DNA Isolation Kit has been able to extract all host DNA including mtDNA from difficult samples like stool samples. Please contact our technical support team at support@norgenbiotek.com and ask for reference publications.
Magnetic Bead System
If the magnetic beads were accidently pipetted up with the supernatant, the pipette tip was placed too close to the magnetic beads while pipetting. Simply return the magnetic beads and the supernatant back into the sample well. Mix well, and place the plate back onto the magnetic separation plate for the specified time. Carefully remove the supernatant without touching the magnetic beads
A low genomic DNA yield may be caused by the following:
- Incomplete lysis of cells.
Ensure that Lysis Additive A is added. Also incubation at 65°C may result in increased yields. - Amount of magnetic beads added was not sufficient.
Ensure that the magnetic bead suspension is mixed well prior to use to avoid any inconsistency in DNA isolation. - DNA concentration in the stool sample being used is low.
Some stool samples contain very little target DNA. This varies from individual to individual based on numerous variables. Incubation at 65°C may result in increased yields.
If the DNA does not perform well in downstream applications, It may be due to one or more of the following:
- DNA was not washed with 70% Ethanol.
Traces of salt from the binding step may remain in the sample if the magnetic beads are not washed with 70% Ethanol. Salt may interfere with downstream applications, and thus must be washed from the magnetic beads. - Ethanol carryover.
Ensure that the drying step after the 70% ethanol wash steps is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications
If RNA is present in eluted DNA, it's because RNA is coeluted with the DNA. Carry out a digestion with RNase A on the elution if the RNA present will interfere with downstream applications. Refer to manufacturer’s instructions regarding amount of enzyme to use, optimal incubation time and temperature.
Citations
| Title | Virological response to nucleos(t)ide analogues treatment in chronic hepatitis B patients is associated with Bacteroides-dominant gut microbiome |
| Citation | eBioMedicine 2024. |
| Authors | Saisai Zhang a f, Hau-Tak Chau a f, Hein Min Tun b c, Fung-Yu Huang a, Danny Ka-Ho Wong a d, Lung-Yi Mak a d, Man-Fung Yuen a d, Wai-Kay Seto |
| Title | Fermented calcium butyrate supplementation in post-peak laying hens improved ovarian function and tibia quality through the "gut-bone" axis |
| Citation | Animal Nutrition 2024. |
| Authors | Huaiyong Zhang a b 1, Yongshuai Wang a 1, Yilu Wang a, Bin Wei a, Leilei Wang a, Minh Tu Nguyen c, Xiangyun Lv d, Yanqun Huang a, Wen Chen a |
| Title | Gut Microbiome Disruption Following SARS-CoV-2: A Review |
| Citation | Microorganisms 2024. |
| Authors | Assunta Sartor |
| Title | In vitro screening of the impact of dietary prebiotic components, probiotic strains, and their symbiotic combinations on colonic microbiota in children with cystic fibrosis |
| Citation | Food & function journal 2024. |
| Authors | Jazmín Viteri-Echeverría, aAnaAndrés,a Joaquim Calvo-Lerma, b Ana Heredia,a Jorge García-Hernándezc and Andrea Asensio-Grau |
| Title | Diet of non-breeding leach's storm-petrels (Hydrobates leucorhous) in the sub-polar frontal zone of the North Atlantic |
| Citation | Marine Biology 2024. |
| Authors | Mónica C. Silva, Paulo Catry, Jason Newton, Vera L. Nunes & Ewan D. Wakefield |
| Title | Sex differences in neuromuscular and biological determinants of isometric maximal force |
| Citation | Acta Physiologica 2024. |
| Authors | Gaia Giuriato1,2 | Maria Grazia Romanelli1 | Desirée Bartolini3 | Gianluca Vernillo4,5 | Anna Pedrinolla1,6 | Tatiana Moro7 | Martino Franchi7 | Elena Locatelli1 | Mehran Emadi Andani1 | Fabio Giuseppe Laginestra1,8 | Chiara Barbi1 | Gloria Fiorini Aloisi1 | Valentia Cavedon1 | Chiara Milanese1 | Elisa Orlandi1 | Tonia De Simone1 | Stefania Fochi1 | Cristina Patuzzo1 | Giovanni Malerba1 | Paolo Fabene1 | Massimo Donadelli1 | Anna Maria Stabile9 | Alessandra Pistilli9 | Mario Rende9 | Francesco Galli3 | Federico Schena1 | Massimo Venturelli |
| Title | The effect of Ajwa dates extract (Phoenix dactylifera L.) on gut microbiota of Sprague Dawley rats induced by a high-fat diet |
| Citation | Food Research 2024. |
| Authors | ,*Karim, M., 2Taslim, N.A., 2Bukhari, A., 3Hamid, F., 4 Idris, I. and 5 Sanusi, H |
| Title | The Age Specific Microbiome of Children with Milk, Egg, and Peanut Allergy |
| Citation | Annals of Allergy, Asthma & Immunology 2024. |
| Authors | Punita Ponda MD 1 2, Jane E. Cerise PhD 3, Brianne Navetta-Modrov MD 4, Jamie Kiehm MD 5, Grace M. Covelli MD 6, Jared Weiss MD 7, Annette T. Lee PhD 8 |
| Title | Carriage of Clostridium perfringens in domestic and farm animals across the central highlands of Colombia: implications for gut health and zoonotic transmission |
| Citation | Veterinary Research Communications 2024. |
| Authors | Anny Camargo, Luisa Páez-Triana, Diego Camargo, Diego García-Corredor, Martin Pulido-Medellín, Milena Camargo, Juan David Ramírez & Marina Muñoz |
| Title | The gastrointestinal antibiotic resistome in pediatric leukemia and lymphoma patients |
| Citation | Frontiers in Cellular and Infection Microbiology 2023. |
| Authors | Tamara MacDonald, Katherine A. Dunn, Jane MacDonald, Morgan G.I. Langille, Johan E. Van Limbergen, Joseph P. Bielawski and Ketan Kulkarni |