Is it possible that your COVID-19 workflow is not as accurate as you may think?
At the start of the COVID-19 pandemic, researchers and laboratories scrambled to develop SARS-CoV-2 test kits in mass quantity. Through much trial and error, standard testing methods were determined and widely accepted around the world. However, what if there was a more accurate testing method than the current standard methods?
The world needs solutions for mass testing to effectively rule out false negatives, ensuring safety for everyone. If you keep reading you'll learn that there may be more accurate COVID-19 testing methods available.
COVID-19 False Negatives
False Negatives are one of the greatest challenges that researchers and clinicians face with the current standard viral RNA extraction methods for SARS-CoV-2.
 When a sample is collected, via nasopharyngeal swab or other sample collection method, it is then stored in Viral Transport Media (VTM) or Universal Transport Media (UTM). This is where the challenge begins. The media often fails to preserve viral RNA efficiently, as it only stabilizes viral RNA in clinical samples for up to 3 days at room temperature. This leads to rapid degradation/fragmentation of the viral RNA. In addition, VTM/UTM does not render the sample noninfectious for safe handling.
When a sample is collected, via nasopharyngeal swab or other sample collection method, it is then stored in Viral Transport Media (VTM) or Universal Transport Media (UTM). This is where the challenge begins. The media often fails to preserve viral RNA efficiently, as it only stabilizes viral RNA in clinical samples for up to 3 days at room temperature. This leads to rapid degradation/fragmentation of the viral RNA. In addition, VTM/UTM does not render the sample noninfectious for safe handling.
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
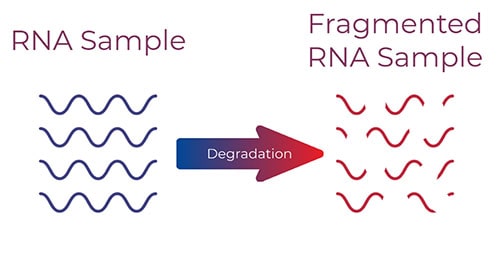 Why does it matter if the viral RNA is fragmented?
Why does it matter if the viral RNA is fragmented?
The current standard for RNA extraction (silica magnetic bead-based technology) cannot efficiently capture highly fragmented RNA. This is due to the bias of silica-based technology towards binding larger RNA sequences. If the fragmented viral RNA is not fully captured, the likelihood to yield false negative COVID-19 results in qRT-PCR based assays is significantly higher.
Comparative Study for COVID-19 RNA Extraction Methods
 This inconsistency with viral RNA extraction methods in standardized COVID-19 workflows led our team to conduct a study to compare our extraction method against silica magnetic bead-based technology. Our goal was to determine if there were any differences in the RNA extraction methods in terms of which method had the highest sensitivity to capture fragmented SARS-CoV-2 viral RNA. 44 clinical nasopharyngeal swab samples were procured from Boca Biolistics (FL, USA). Using the FDA/EUA approved PerkinElmer® SARS-CoV-2 Real-time RT-PCR Assay, the samples were pre-screened to confirm the 44 samples were SARS-CoV-2 positive.
This inconsistency with viral RNA extraction methods in standardized COVID-19 workflows led our team to conduct a study to compare our extraction method against silica magnetic bead-based technology. Our goal was to determine if there were any differences in the RNA extraction methods in terms of which method had the highest sensitivity to capture fragmented SARS-CoV-2 viral RNA. 44 clinical nasopharyngeal swab samples were procured from Boca Biolistics (FL, USA). Using the FDA/EUA approved PerkinElmer® SARS-CoV-2 Real-time RT-PCR Assay, the samples were pre-screened to confirm the 44 samples were SARS-CoV-2 positive.
Silica OR Silicon Carbide?
The efficiency of 2 RNA extraction methods were investigated for their accuracy in detecting SARS-CoV-2 viral RNA in the 44 clinical samples mentioned previously.
The first method was the most common method for extracting viral RNA, traditional silica magnetic bead-based technology (Quick-DNA/RNA Viral MagBead by Zymo Research) for viral RNA extraction. The second method was a silicon carbide-based extraction method (Saliva/Swab RNA Purification Kit by Norgen).
What is the Best RNA Purification Technology?
 Now this is where things get interesting.
Now this is where things get interesting.
The RNA was extracted using both methods and ran on a qRT-PCR assay using the 2019-nCoV TaqMan RT-PCR Kit (Cat. TM67100), targeting 3 genes (N1, N2 and RP) as recommended by the CDC.
When the qRT-PCR data from the two methods was compared, we found that the silica magnetic bead-based extraction method resulted in a 25% false negative rate for the true positive SARS-CoV-2 samples (Figure 1). On the other hand, the silicon carbide-based extraction method resulted in a 100% true positive rate for the SARS-CoV-2 samples and had more consistent Ct values when compared to silica magnetic bead-based methods These results made it clear that false negative results could be avoided by using our silicon carbide (SiC)-based technology instead of the traditional Silica-based technology.
| Silicon Carbide-based Technology (Norgen Biotek) |
Silica Magnetic Bead-based Technology | |||
| SARS-CoV-2 Positive | 44 | 33 | ||
| False Negative | 0 | 11 | ||
| True Positive | False Negative | True Positive | False Negative | |
| 100% | 0% | 75% | 25% | |
How to Improve your RNA Extraction Workflow for COVID-19 Testing
Silicon carbide is a novel RNA extraction technology that has superior RNA binding affinity - specifically to small, highly degraded RNA fragments, such as viral RNA. Silicon carbide has a uniform binding affinity to all sequences of RNA, without ANY bias to GC content or size. Additionally, silicon carbide does not require any carrier RNA or hazardous chemicals like phenol/chloroform.
Why have you not heard of Silicon Carbide-based RNA extraction before?
 Silicon carbide is Norgen Biotek's patented technology for RNA extraction. Based in Thorold, Ontario, Norgen was founded in 1998 and has been utilizing silicon carbide-based technology to drastically improve RNA yield and quality from challenging samples for our loyal customers. Currently our silicon carbide (SiC) technology is being utilized in COVID-19 FDA approved workflows for 24-hour public testing.
Silicon carbide is Norgen Biotek's patented technology for RNA extraction. Based in Thorold, Ontario, Norgen was founded in 1998 and has been utilizing silicon carbide-based technology to drastically improve RNA yield and quality from challenging samples for our loyal customers. Currently our silicon carbide (SiC) technology is being utilized in COVID-19 FDA approved workflows for 24-hour public testing.
What does this mean for YOU?
It is imperative that testing laboratories are utilizing the most accurate technologies to ensure reliable reporting of SARS-CoV-2 virus infection. The standardized silica magnetic bead-based extraction method may not be the most accurate method for testing for SARS-CoV-2, as seen in the study producing a 25% false negative rate.
It is time for the quality of the entire screening workflow to be evaluated - from the collection of biological samples, to viral RNA extraction, to SARS-CoV-2 detection - to ultimately protect the health and safety of the global population.
Try a sample of Norgen's silicon carbide-based RNA extraction kit today!
View Complete COVID-19 Workflow
Have additional questions? Comment below!