1 Sample Collection & Preservation
Saliva RNA Collection & Preservation Device
New Price
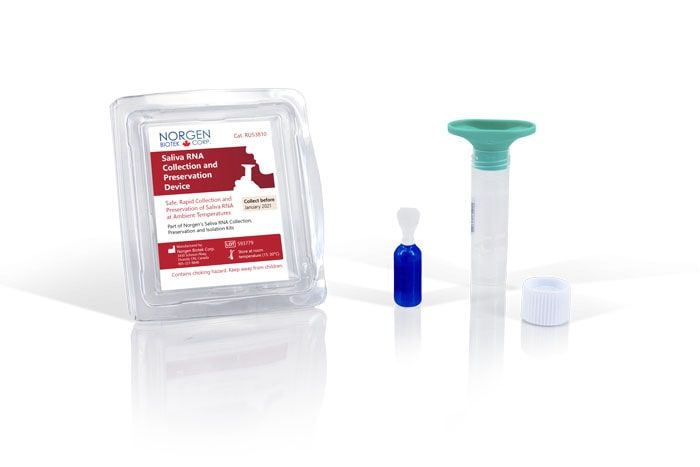
Norgen's Saliva RNA Collection and Preservation Devices Dx are designed for 1) safe and non-invasive saliva collection and 2) preservation of RNA in saliva samples at ambient temperature. Each of the 50 Saliva RNA Collection and Preservation Devices Dx consists of 3 components: (1) Saliva Collection Funnel and Collection Tube, (2) Collection Tube Cap, and (3) Norgen's Saliva RNA Preservative contained within a sealed squeezable ampoule. Saliva samples are collected by spitting inside the Collection Funnel which has been assembled with the Collection Tube. After collecting the required volume of saliva the Collection Funnel is removed and the contents of the Preservative Ampoule are then added and mixed with the collected saliva. The Saliva Collection Tube is subsequently sent to the laboratory for RNA isolation and analysis. RNA can be isolated from the preserved saliva samples using Norgen's Saliva/Swab RNA Purification Kits Dx (Cat# 69100/69300). Each of Norgen's Collection Tubes is labeled with a unique serial number that can be used for secure and anonymous tracking of the sample. The saliva RNA in preserved samples is stable for up to 2 months at room temperature. This kit is ideal for collecting and preserving RNA samples for in vitro diagnostic use for medical purposes.
- Non-invasive collection procedure — just spit, twist, test
- Viral inactivation for safe transport & handling
- Tested on SARS-CoV-2 virus
- Inactivates virus within 5 minutes of mixing
- CE-IVD marked version available
- Authorized for sale by Health Canada
- Suitable for self-collection of saliva samples supervised by a trained individual in the collection procedure by watching the training video and reading over the collection instructions.
Total Nucleic Acid Preservation Tubes
Discount
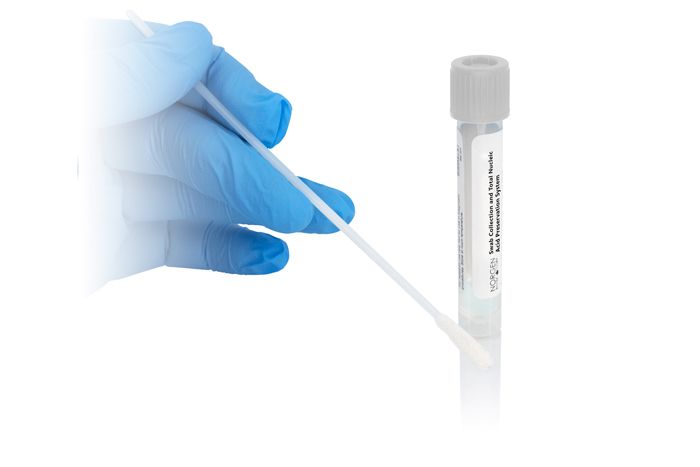
Norgen's Total Nucleic Acid Preservation Tubes Dx are designed for ambient preservation and transport of total nucleic acids (DNA and RNA) from samples collected using a swab. The Total Nucleic Acid Preservation Tubes contain Norgen's Total Nucleic Acid Preservative in a liquid format. The user simply collects the specimen using a sterile nylon flocked swab (not provided), and then transfers the swab into the Total Nucleic Acid Preservative. The Preservative prevents the growth of Gram-negative and Gram-positive bacteria and fungi, and also inactivates viruses allowing the resulting non-infectious samples to be handled and shipped safely. In addition, the Total Nucleic Acid Preservative eliminates the need to immediately process or freeze samples and allows the samples to be shipped to centralized testing facilities at ambient temperatures. The components of the preservative allow DNA samples to be stored at room temperature for over 4 months and RNA samples to be stored at room temperature for over 2 months. This kit is ideal for collecting and preserving nasal and oral swab samples for in vitro diagnostic use for medical purposes. Norgen's Total Nucleic Acid Preservation Tubes Dx are intended for use by professional users such as technicians, physicians and biologists experienced and trained in molecular biological techniques. Norgen's Total Nucleic Acid Preservation Tubes Dx do not provide a diagnostic result. It is the sole responsibility of the user to use and validate these devices in conjunction with a downstream in vitro diagnostic assay.
- No need to immediately process samples
- Total DNA, including viral DNA, preserved at room temperature over 4 months
- Total RNA, including viral RNA, preserved at room temperature over 2 months
- Inactivate microorganisms including bacteria, fungi, yeast and viruses
- Ship swab samples at room temperature safely
- Compatible with most DNA and Viral RNA isolation methods
- CE-IVD marked version available
- Authorized for sale by Health Canada
2 Viral RNA Isolation
Saliva/Swab RNA Purification Kits
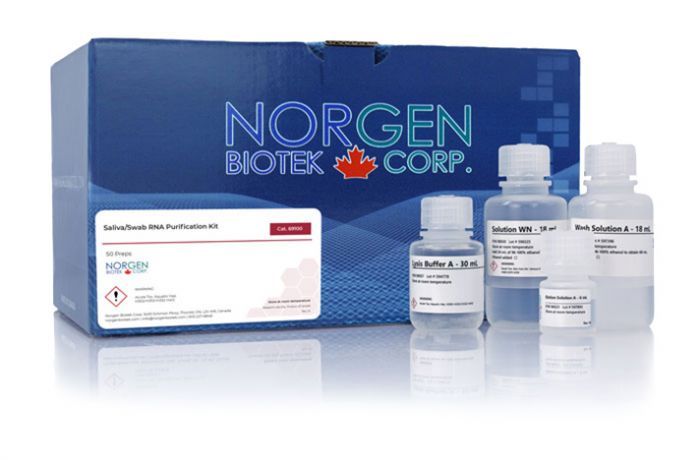
Norgen's Saliva/Swab RNA Purification Kit provides a rapid method for the purification of total RNA from non-preserved saliva and nasal/throat swabs, and from preserved saliva collected on Norgen's Saliva RNA Collection and Preservation Devices (Cat. RU53800) or preserved swabs collected in Norgen's Total Nucleic Acid Preservative Tubes (Cat. 69200). Purification is based on using Norgen's proprietary resin separation matrix. RNA is preferentially purified from other cellular components such as proteins, without the use of phenol or chloroform. The kit allows the purification of total RNA, including viral and bacterial RNA, irrespective of size or GC content. The purified RNA is eluted in an Elution Solution that is compatible with all downstream applications including PCR, qPCR, methylation-sensitive PCR and Southern Blot analysis, microarrays, and NGS.
- Isolate high quality total RNA, including viral RNA, from fresh and preserved saliva and swab samples
- Fast and easy processing using rapid spin-column format
- Isolate total RNA, from large rRNA down to microRNA (miRNA)
- No phenol or chloroform extractions
- Very sensitive & linear down to a few cells/viral copies without the need for carrier RNA
- Buffer chemistry inactivates viruses including SARS-CoV-2
- High throughput 96-well plate format for rapid purifications is also available
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
- Authorized for sale by Health Canada
- CE-IVD marked version available
Total RNA Purification Kits

Norgen's Total RNA Purification Kit Dx provides a rapid method for the isolation and
purification of total RNA from tissue samples, blood, plasma, serum, saliva, bacteria,
yeast, fungi and viruses for subsequent
in vitro
diagnostic use.
This kit is designed to be used with any downstream application employing enzymatic
amplification or other enzymatic modifications of RNA followed by signal detection or
amplification. Extract high quality and purity RNA with excellent RIN values and
A260/A280 suitable for downstream applications including qRT-PCR, RT-PCR, microarrays,
NGS and more. Any diagnostic results generated using the RNA isolated with Norgen's
Total RNA Purification Kit Dx in conjunction with an
in vitro
diagnostic assay should be interpreted with regard to other clinical or laboratory
findings.
- Extract high quality & purity total RNA including miRNA
- No phenol step required
- Bind & elute all RNA irrespective of size or GC content, without bias
- Very sensitive & linear down to a few cells without the need for carrier RNA
- Convenient & fast spin column format
- Isolate from a wide variety of specimens
- Purified RNA is suitable for a variety of downstream applications
- Buffer chemistry inactivates viruses including SARS-CoV-2
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
- Authorized for sale by Health Canada
- CE-IVD marked version available
3 RT-PCR Detection
COVID-19 TaqMan RT-PCR Kit (N/ORF1ab genes)
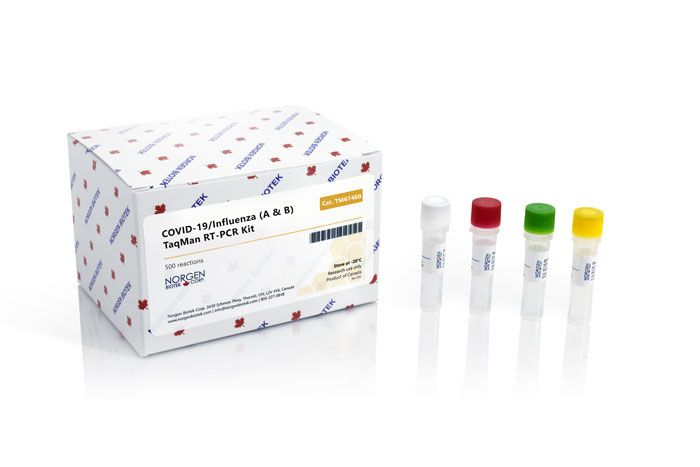
Norgen's COVID-19/Influenza (A & B) TaqMan RT-PCR Kit includes 2X One-Step RT-PCR
Master Mix, a primer/probe mix, a positive control and a negative control
(nuclease-free water). The primer/probe mix contains SARS-CoV-2 detection target; N
(Nucleoprotein), Influenza A detection target; M gene and Influenza B detection
target; HA (Hemaglutinin) and the human RNase P transcript as an internal control
target to monitor for PCR inhibition, and to validate the quality of the sample and
the detection result. The provided N/Inf A & B/RP Positive Control contains an in
vitro RNA transcript for the SARS-related target gene (N Gene), and genes associated
with Influenza A (M gene) and Influenza B (HA Gene), as well as the human RP gene
(internal control).
- Multiplex RT-PCR system detects two SARS-CoV-2 specific genes (N gene and ORF1ab) in a single one step RT-PCR reaction
- Primer & Probe Mixes contain all N gene, ORF1ab and human RP assays
- Positive Control contains the three SARS-related target genes: N gene, ORF1ab gene as well as the human RP (internal control).
- Convenient ready-to-use 2X One-Step RT-PCR Master Mix
- CE-IVD marked version availble
COVID-19 TaqMan RT-PCR Kit (E/RdRP genes)
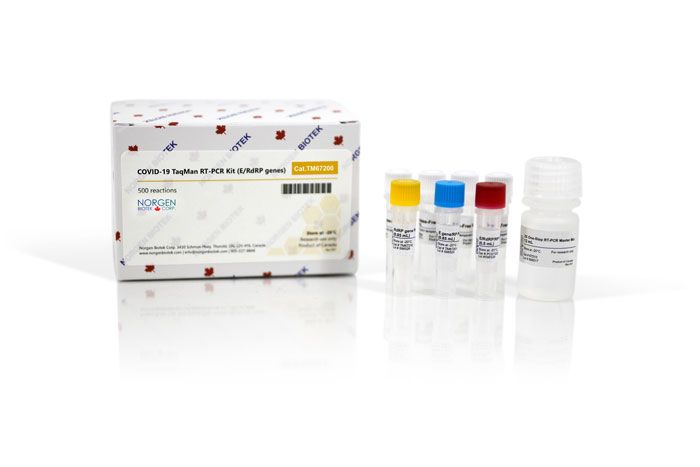
Norgen's COVID-19 TaqMan RT-PCR Kit (E/RdRP genes) Dx is an in vitro diagnostic test for the qualitative detection of SARS-CoV-2 specific RNA using a multiplexed TaqMan® fluorescence detection assay (FAM and HEX/VIC) based on the Charité/Berlin protocol. The assay is designed for use with RNA isolated from nasopharyngeal swabs, oropharyngeal swabs and saliva samples collected from individuals with clinical signs/symptoms related to SARS-CoV-2 infection for in vitro diagnostic use.
- SARS-CoV-2 detection based on the Charité/Berlin protocol (E/RdRP genes) recommended by WHO
- Primer & Probe Mixes contain all E gene/RP and RdRP gene assays in individual tubes
- All assays are pre-mixed to the working concentrations recommended by the Charité/Berlin protocol
- Positive Control contains the three SARS-related target genes: E gene, RdRP gene as well as the human RP (internal control)
- Convenient ready-to-use 2X One-Step RT-PCR Master Mix
- Authorized for sale by Health Canada
- CE-IVD marked version available
2019-nCoV TaqMan RT-PCR Kit
Norgen's 2019-nCoV TaqMan RT-PCR Kit is designed for the detection of SARS-CoV-2 specific RNA in a real-time RT-PCR based on the use of TaqMan technology. This kit is designed for research use only and not for use in diagnostic procedures. The detection of SARS-CoV-2 specific RNA is based on TaqMan one-step RT-PCR providing a simple, reliable and rapid result for the detection of SARS-CoV-2 infection. Norgen's 2019-nCoV TaqMan RT-PCR Kit includes a PCR control to monitor for PCR inhibition, and to validate the quality of the sample and the detection result. The 2019-nCoV TaqMan RT-PCR Kit comprises Master Mix for the target and PCR control detection, 3 target Primer & Probe Mixes, as well as a positive control and a negative control (nuclease-free water) to confirm the integrity of the kit reagents.
- CE-IVD marked in accordance with EU Directive 98/79/EC
- Authorized for sale by Health Canada
- Fits into in vitro diagnostic workflows
- COVID-19 detection based on the assays and protocols developed by the CDC
- Primer & Probe Mixes contain all 3 CDC developed assays in individual tubes
- All assays are premixed to the working concentration recommended by the CDC
- Positive Control contains 2 nCoV nucleocapsid target gene RNA (N1,N2) and RNase P (internal control) which are compatible with the CDC primer/probe set
- Convenient ready-to-use 2X One-Step RT-PCR Master Mix
COVID-19/Influenza (A & B) TaqMan RT-PCR Kit

Norgen's COVID-19/Influenza (A & B) TaqMan RT-PCR Kit includes 2X One-Step RT-PCR Master Mix, a primer/probe mix, a positive control and a negative control (nuclease-free water). The primer/probe mix contains SARS-CoV-2 detection target; N (Nucleoprotein), Influenza A detection target; M gene and Influenza B detection target; HA (Hemaglutinin) and the human RNase P transcript as an internal control target to monitor for PCR inhibition, and to validate the quality of the sample and the detection result. The provided N/Inf A & B/RP Positive Control contains an in vitro RNA transcript for the two SARS-related target genes: N gene, ORF1ab gene as well as the human RP gene (internal control).
- Multiplex RT-PCR system detects SARS-CoV-2 specific gene (N gene), Influenza A (M gene) and Influenza B (HA gene) in a single one step RT-PCR reaction
- Primer & Probe Mixes contain all N gene, M gene, HA gene and human RP assays
- Positive Control contains the SARS-related target genes: N gene, Influenza A target gene (M gene), Influenza B target gene (HA gene) as well as the human RP (internal control)
- Convenient ready-to-use 2X One-Step RT-PCR Master Mix included in kit
- Manufactured under ISO 13485:2016 for research use only
- CE-IVD marked version pending
Discounted Bundles
We have exclusive deals and discount if you pair the products togehter. Fill out the form below and our sales presentative will process your discounted bundle soon!
Discounted Bundles
Thank you!
A sales presentative will process your discounted bundle and will be in touch soon!