In this blog, you’re going to learn what makes saliva a viable alternative to nasal and oral swabs for the detection of SARS-CoV-2.
This post includes statistics on real-life examples of SARS-CoV-2 being isolated from different sample types, including saliva and swabs, showing high sensitivity, longevity, and more.
If you want to learn why saliva is a great alternative to the current gold standard of testing, nasopharyngeal swabs, you’ll really love this blog.
So, let’s get right into it!
Why saliva testing?
- Saliva testing can help with the global shortage of swabs for sampling and increase testing frequency
- Saliva is as sensitive as nasopharyngeal swabs for SARS-CoV-2 detection in COVID19 patients
- Saliva sampling reduces the risk and challenges associated with nasopharyngeal swabs, which require trained personnel and show higher variability in downstream screening
In the months following the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) outbreak, nasopharyngeal and oral swabs were initially used to collect samples needed for testing. Widespread testing using these traditional sampling methods quickly proved to be challenging as shortages of critical testing supplies such as swabs and reagents were reported by health care practitioners across North America (Pedersen, 2020; Webbet and Jewett, 2020). In addition to these shortages, nasopharyngeal and oral swabs are difficult to perform properly for untrained personnel and can be quite uncomfortable for the individual providing the sample.This was troubling as nasopharyngeal swabs, in particular, were considered the “gold standard” in SARS-CoV-2 screening procedures.
 Spurred by these challenges, several research groups began exploring the utility of other analytes that could be used in the detection of COVID19. Since March, there has been a growing body of evidence demonstrating that viral titers in saliva are similar to that found in swabs (Azzi et al., 2020; Sapkota et al., 2020; To KK et al., 2020; Williams et al., 2020; Health Canada, 2020). One of the largest prospective specimen type comparisons to date, was led by researchers at University of Utah, who analyzed more than 1,100 specimens from 368 volunteers at a drive-through testing site from late May through June (Hansen et al., 2020). The researchers compared test results from self-collected saliva samples and nasopharyngeal swabs healthcare providers collected from the volunteers. Discrepant results across specimens collected from the same patient prompted repeat testing using a second PCR-based assay. The study showed that SARS-CoV-2 was detected in at least two specimen types in 90% of the patients who tested positive for the virus. The study also showed that self-collected nasal swabs, when used alone, missed nearly 15% of infections. Other studies, including one from the Yale School of Public Health, have reached similar conclusions (Wyllie et al., 2020).
Spurred by these challenges, several research groups began exploring the utility of other analytes that could be used in the detection of COVID19. Since March, there has been a growing body of evidence demonstrating that viral titers in saliva are similar to that found in swabs (Azzi et al., 2020; Sapkota et al., 2020; To KK et al., 2020; Williams et al., 2020; Health Canada, 2020). One of the largest prospective specimen type comparisons to date, was led by researchers at University of Utah, who analyzed more than 1,100 specimens from 368 volunteers at a drive-through testing site from late May through June (Hansen et al., 2020). The researchers compared test results from self-collected saliva samples and nasopharyngeal swabs healthcare providers collected from the volunteers. Discrepant results across specimens collected from the same patient prompted repeat testing using a second PCR-based assay. The study showed that SARS-CoV-2 was detected in at least two specimen types in 90% of the patients who tested positive for the virus. The study also showed that self-collected nasal swabs, when used alone, missed nearly 15% of infections. Other studies, including one from the Yale School of Public Health, have reached similar conclusions (Wyllie et al., 2020).
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
In light of this evidence, SARS-CoV-2 tests that utilize saliva as an analyte began to emerge as alternatives to traditional tests that utilize swabs. These tests could be a component of the robust mass surveillance program necessary for controlling the COVID19 pandemic as they require different supplies than the standard swabs, which could help ease medical supply shortages. Additionally, saliva samples can be self-collected, significantly reducing the risk of exposure to SARS-CoV-2 for health care workers. Saliva samples are also easier to obtain and less invasive than standard nasopharyngeal and oral swabs.
Figure 1. SARS-CoV-2 RNA Titers in Saliva Specimens and Nasopharyngeal Swab Specimens (Wyllie et al., 2020).
Saliva-Based Testing in Canada
In the United States, the FDA has 5 saliva-based diagnostic tests approved for COVID19 testing. While Health Canada has received two applications for tests that use saliva samples, these have yet to be approved, at the time of this report. It should be noted, however, that provincial and territorial laboratories which are regulated under provincial jurisdiction can develop their own diagnostic tests that utilize saliva samples.
Unlike nasal swabs, the non-invasive nature of saliva collection devices removes the requirement for Health Canada approval for their use in Canada. British Columbia recently approved the use of “swish-and spit” sampling, which involves gargling a cup of salt water, for children from kindergarten up to Grade 12. The samples are then submitted to the lab for COVID19 screening. In a recent press briefing, Premier Doug Ford stressed the importance of using similar saliva-based tests to alleviate screening delays and announced that three Toronto hospital assessment centres would begin offering people the option of spitting in a cup for their COVID19 test. The main drawback of using a simple saline solution to collect saliva is that it leaves shed viral cells active. Mishandling of samples by anyone, either at the point-of-care or the laboratory, could potentially leave handlers exposed to live virus. The use of lytic preservatives in the collection, storage and transport of saliva samples eliminates this concern. Some notable manufacturers supplying saliva collection devices with virus inactivating preservatives include the Ontario-based manufacturers, Norgen and OraSure. Both companies have ramped up production of these devices in response to evidence suggesting that saliva is a viable alternative to nasal and oral swabs.
The availability of multiple types of diagnostic tests for SARS-CoV-2 helps lower costs for the healthcare system by reducing the likelihood of shortages of any one (or few) diagnostic kits. Similarly, utilizing multiple analytes, particularly ones that allow for self-collection could contribute to reduced costs as they have fewer components (i.e. no swabs) and most do not require the presence of a trained healthcare practitioner for specimen collection.

Routine Mass Screening
Saliva offers a number of advantages over nasopharyngeal swabs when considering the aforementioned criteria for mass screening efforts. For one, it does not require a certified swab and collection receptacle and does not necessarily have to be obtained by a skilled healthcare provider, therefore it would significantly reduce screening costs. Additionally, because nasopharyngeal sampling requires a swab being inserted into the back of the nares, it can cause irritation that could promote sneezing and coughing. Thus, the non-invasive collection of saliva is safer as it protects healthcare workers from being inadvertently exposed to potentially infectious droplets. Especially if the saliva is preserved in a virus inactivating solution upon collection instead of the standard viral transport medium (VTM) or saline solution. Lastly, the use of these preservatives eliminates the need for cold chain storage and transport, as they offer nucleic acid stabilization at room temperature (RNA for 2 months and DNA for 2 years).
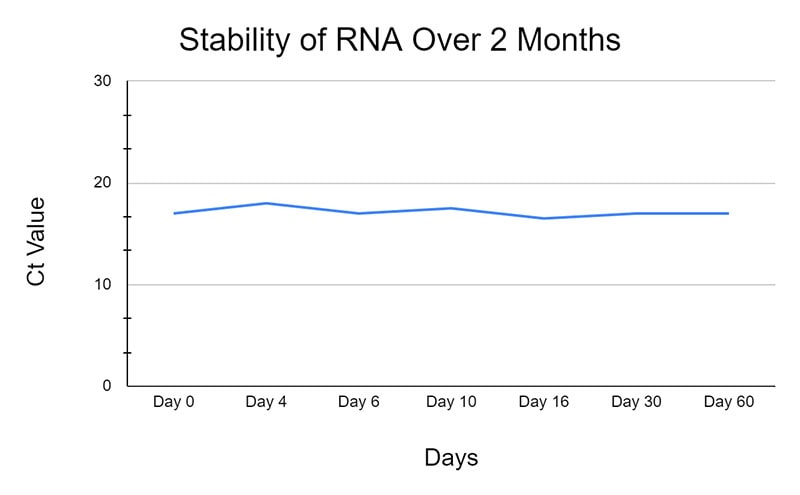
Figure 1: Stability of RNA and DNA (Norgens Total Nucleic Acid Preservation Tubes)
Collection of nasopharyngeal swabs has also been associated with variable and false negative test results due to the technical difficulties of taking a proper swab. Interestingly, there is evidence that different types of samples collected may yield different results in the same individual being tested (Williams et al., 2020; Azzi et al., 2020). This finding makes it difficult to directly compare test results that utilize different sampling techniques. There is also evidence that viral loads of SARS-CoV-2 in saliva are highest during the initial phase of infection which gradually decline over time (To KK et al., 2020) This is consistent with other data that suggests pharyngeal viral shedding is highest in the first week of symptoms (Wölfel et al., 2020). It is therefore likely important to use saliva as an analyte for patients showing early signs of infection or routine screenings. The ability to test large groups of people with or without COVID19 symptoms is a necessary part of a public health strategy to manage the pandemic.
Summary
- The benefits of using saliva sampling varies depending on how the sampling is deployed. As samples can be self-collected, saliva sampling does not require the same degree of direct contact between a healthcare worker and an individual suspected of having COVID19 that a traditional nasopharyngeal swab requires. This is a potential benefit to health care workers as they would have less risk of exposure to the SARS-CoV-2 pathogen.
- Another group that might benefit from saliva-based screening would be individuals who are symptomatic for COVID19 and require testing for a diagnosis. Increased availability of saliva collection devices could result in increased testing capacity, which may lead to shorter waiting times for testing.
- Providing a saliva sample is more comfortable and less invasive than a nasopharyngeal swab, which might decrease the stress experienced by an individual being tested for SARS-CoV-2. The ability to test large groups of people with or without COVID19 symptoms is a necessary part of a public health strategy to manage the pandemic.