March is Multiple Myeloma Month
March is Multiple Myeloma Month, which aims to raise awareness about this blood cancer that affects 1 in 100 American men (1/130 women).1 Multiple Myeloma cannot be cured, however, treatment can slow the progression and minimize symptoms. Since 1990, the incidence of Multiple Myeloma has risen by 126% globally, and over 40% in the US. Although global mortality has risen by 94%, mortality in the US has decreased over this same period. With the introduction of new targeted therapies and transplant techniques, the 5 year survival rate in the US has more than doubled over the past decades. Risk factors for Multiple Myeloma include age (average age of diagnosis is 69 years), race (African Americans are over double as likely to be diagnosed), sex (men are at a 1.5x risk), and family history.
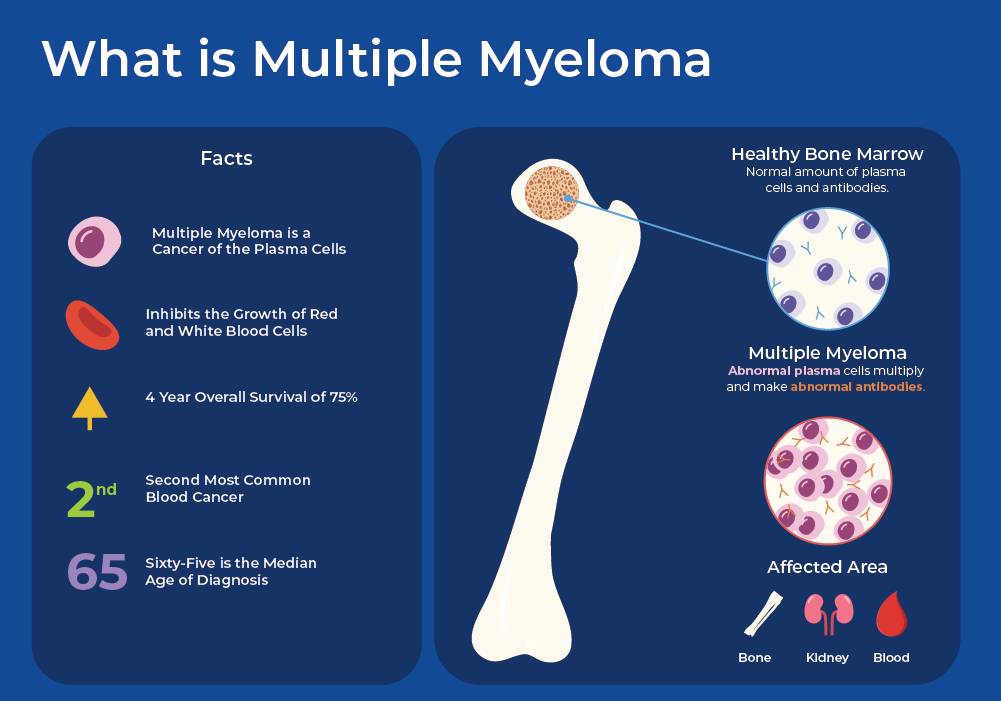
What is Multiple Myeloma?
Multiple Myeloma (MM) is a biologically heterogeneous and incurable cancer of the blood. It starts in the bone marrow when there is uncontrolled growth of plasma cells. One of the main functions of plasma is to produce immunoglobulins, or antibodies, which are important for your immune system function. In Multiple Myeloma, plasma cells produce non-functional monoclonal immunoglobulin protein (M proteins). The malignant myeloma cells crowd out the normal cells in the bone marrow that make the red blood cells and other components. Multiple Myeloma (MM) is preceded by a premalignant condition called monoclonal gammopathy of undetermined significance (MGUS), which is defined by the accumulation of M proteins. MGUS can develop into an intermediate stage called smoldering multiple myeloma (SMM), which can ultimately develop the symptomatic stage of Multiple Myeloma, which includes end-organ damage. Multiple myeloma (MM) cells have the potential to proliferate beyond the confines of the bone marrow, leading to the emergence of more aggressive forms like extramedullary multiple myeloma (EMM) or plasma cell leukemia (PCL).
Plasma/Serum Exosome and Free-Circulating RNA Isolation Kits
All-in-one system for the purification of exosomes and the sequential isolation of RNA and free-circulating RNA from plasma/serum samples.
Learn MoreThe transition from MGUS to multiple myeloma (MM) remains elusive. However, genetic alterations are often implicated in disease progression. Moreover, cells within the bone marrow microenvironment play a crucial role in driving MM advancement. These cells, including bone marrow stromal cells, endothelial cells, and other hematopoietic cells, secrete chemokines and other molecules that interact with MM cells, promoting their migration to the bone marrow and facilitating their proliferation and survival. The disease is associated with an unfavorable prognosis, attributed to disease relapse and the emergence of treatment resistance. Chemotherapy, stem cell transplantation, and targeted drug therapy are currently available therapeutic options for the treatment of MM patients, with the aim to improve their quality of life and prolong survival time.
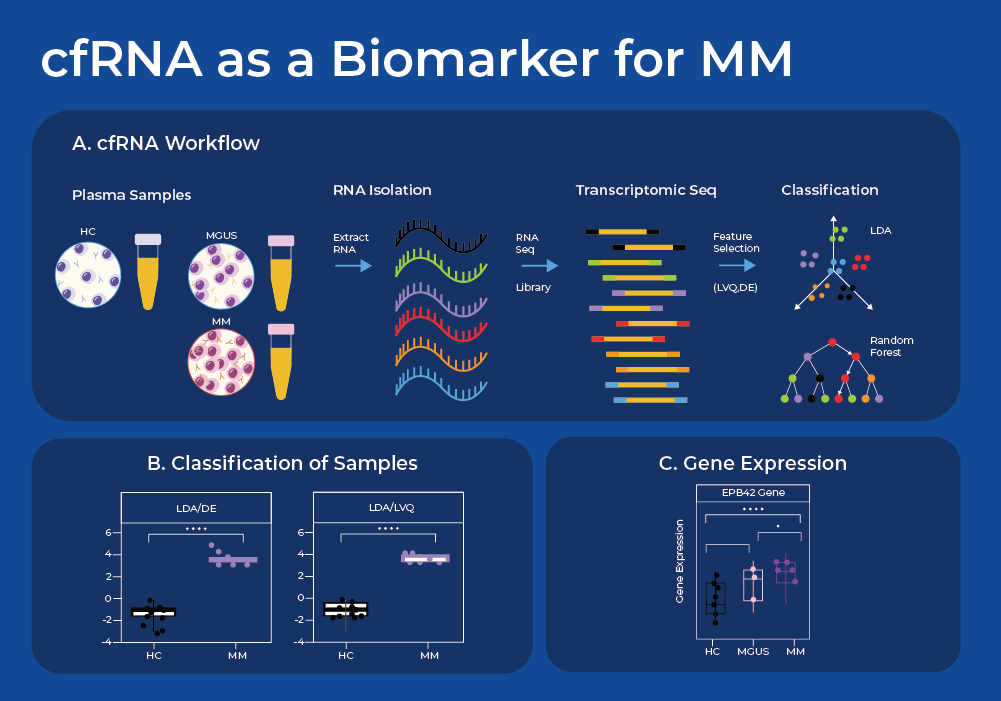
cfRNA as a Biomarker for Multiple Myeloma
Despite advances in cancer treatment, early detection of cancer still provides the highest chance of improving long-term patient survival. Even with blood cancers like multiple myeloma (MM), 95% of patients are diagnosed when cancer has already spread systemically. This late diagnosis results in at least a 20% decrease in 5-year survival rates compared to detection at earlier stages.2 Noninvasive, low cost, and reliable cancer diagnostic assays such as liquid biopsies could greatly benefit patients by facilitating accessibility to early cancer screening.
Cell-free RNA (cfRNA) circulating in the bloodstream originates from cells via active secretion or as a result of apoptosis and necrosis. Thus, analysis of cell-free RNA (cfRNA) in plasma can serve as an indicator of phenotypic changes occurring in both localized cancer sites and the systemic host response, and can indicate the tissue of origin. One recent study used Norgen's plasma/serum circulating and exosomal RNA purification kit to extract cfRNA and perform subsequent RNA sequencing. They sequenced cfRNA from plasma samples of patients with MM, their precancerous conditions (MGUS), and non-cancerous (NC) donors.3
After sequence analysis, they used machine learning models to find combinations of discriminating genes to separate cancerous from non-cancerous individuals. They identified a set of 10 genes whose expression levels can be used to classify plasma samples between the NC, MGUS, and MM donors. Moreover, these genes displayed a gradual increase in cfRNA level from the non-cancer donors through MGUS to MM. When the three group classification model was used, this gene set model resulted in a classification accuracy of 86.8% (18/20 NC, 6/8 MGUS, and 9/10 MM). The fact that 8 of these 10 genes are known to have higher expression in bone marrow supports the biological integrity of the machine learning model. Thus, this study shows proof of concept that a panel of cfRNA sequences may be able to classify a patient's stage of Multiple Myeloma, and offers potential for a minimally invasive and cost-effective method for early cancer detection. Liquid biopsies can also reveal Exosomal RNA and proteins that may be useful biomarkers for MM,4 as has been covered in a previous blog.
Uncovering the Role of ncRNA in Multiple Myeloma
Epigenetic dysregulation has emerged as an important factor in the pathogenesis of multiple myeloma (MM). This includes alterations in histone modification, DNA methylation, and microRNA (miRNA) activity. miRNAs are non-coding short RNAs whose primary function is to inhibit mRNA translation into protein by binding to complementary sequences on RNA molecules. miRNAs profoundly influence various biological processes, including cellular differentiation, metabolism, proliferation, and apoptosis. Dysregulation of miRNAs can disrupt essential cellular functions, contributing to the initiation and progression of various diseases, including cancer.
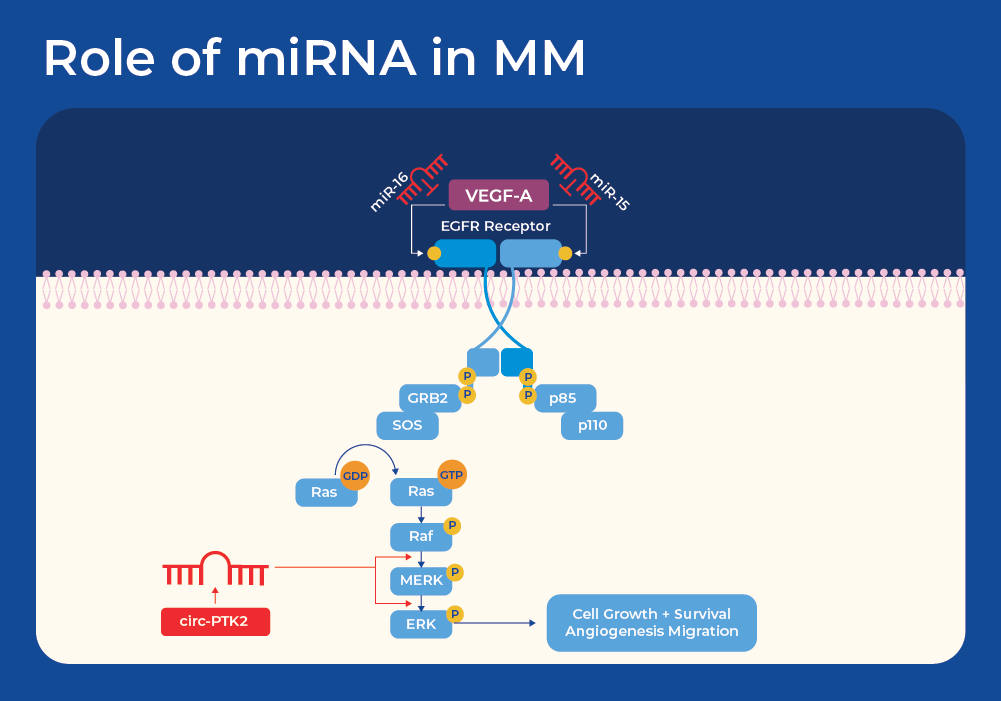
miRNAs in Multiple Myeloma
One known example of the influence of miRNAs on MM is through the EGFR signaling pathway. The epidermal growth factor receptor (EGFR) signaling pathway is a pivotal mechanism governing cell growth, survival, proliferation, and differentiation in mammalian cells, and has been implicated in the development and progression of the majority of cancer types. In addition, vascular endothelial growth factor (VEGF) is an important growth factor involved in angiogenesis, the formation of new blood vessels. EGFR and VEGF share common downstream signaling pathways. Studies have shown that miR-15a/16 may contribute to the development of MM tumors by controlling angiogenesis through the targeting of VEGF-A.5 This knowledge has been harnessed for other cancers as miR-16 mimics are now in a Phase I clinical trial for patients with malignant pleural mesothelioma. Minicells loaded with miR-16-based mimic miRNA and targeted to EGFR are designed to improve the loss of the miR-15 and miR-16 family miRNAs in tumor cells.6
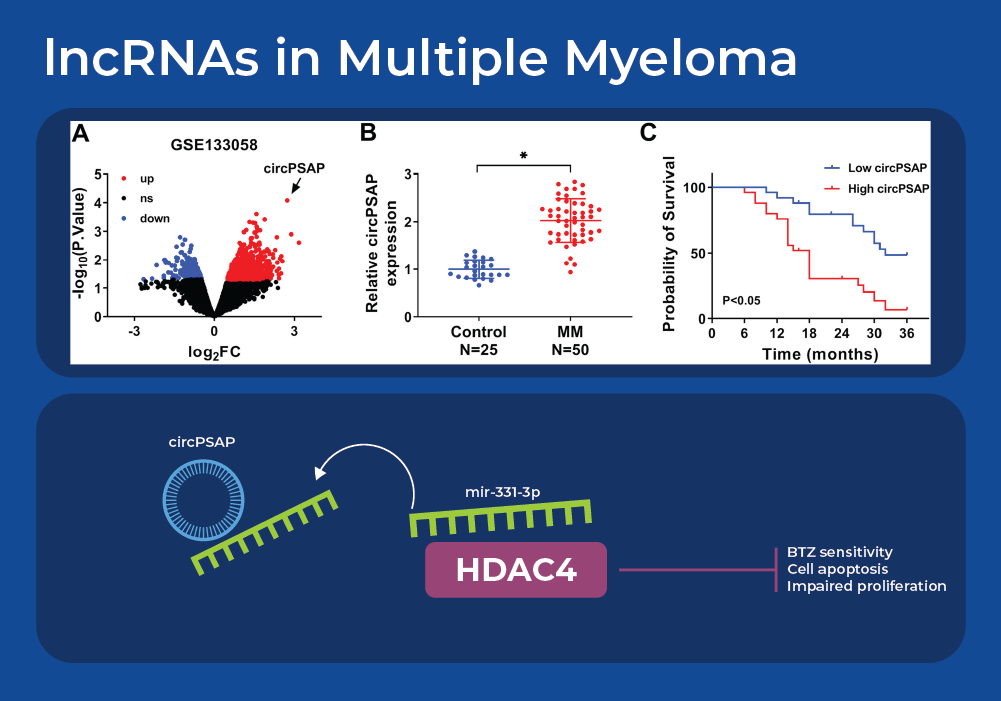
lncRNAs in Multiple Myeloma
Understanding the effect of miRNAs on cancer gets more complicated as we integrate knowledge of long non-coding RNAs (lncRNAs). Recent research is revealing a network of interacting lncRNA, miRNA, and cancer pathways. Analysis of gene expression data sets from multiple myeloma patients identified one highly differentially expressed circular RNA (circRNA) - circPSAP. This was further confirmed in a larger cohort size by using qRT-PCR to analyze bone marrow specimens. Firstly, it was noted that circPSAP was significantly over expressed in patients with MM than the health controls. Moreover, the expression of circPSAP correlated with the survival rate that was predicted using clinical pathological analysis. Bioinformatic analysis identified a miRNA that contains a complementary sequence to circPSAP - miR-331-3p. The cytoplasmic location of this miRNA was examined using Norgen's Cytoplasmic & Nuclear RNA Purification Kit. Further experiments confirmed that histone deacetylase 4 (HDAC4) is the target of miR-331-3p. The HDAC family exerts important activity in the development and Bortezomib (BTZ) resistance of MM. Circular RNA circPSAP functions as an efficient miR-331-3p sponge to regulate proliferation, apoptosis, and bortezomib sensitivity of human MM cells by upregulating HDAC4.7
lncRNAs in drug resistance
Different researchers revealed a similar triad story where lncRNA H19 acted as a competitive endogenous RNA (ceRNA) sponge of miRNA-29b-3p to promote expression of MCL-1. The Myeloid cell leukemia sequence 1 protein (MCL-1) plays a key role in the growth of cancer cells “escaping” drug attacks. Its expression is often implicated in drug resistance. It was demonstrated that H19 inhibited apoptosis of MM cells and promoted BTZ resistance by regulating the translation of MCL-1 through sponging miR-29b-3p. This suggests that H19/miR-29b-3p/MCL-1 may be a novel and promising therapeutic target for coping with drug resistance in MM treatment.8
Single-Cell RNA Sequencing
Research at single-cell resolution has revealed significant inter-patient heterogeneity across the disease spectrum from MGUS to active MM, and even the relapsed/refractory multiple myeloma (RRMM) stage. Single cell RNA sequencing (scRNA-seq) has also revealed that Multiple Myeloma displays high intra-tumor heterogeneity consisting of a mixture of clones and diverse transcriptional programs, which pose both obstacles and opportunities for myeloma therapy.
Recently, researchers applied scRNA-seq to individuals before and after two rounds of Proteasome inhibitor (PI) treatment. After 2 cycles of treatment, each patient exhibited a different extent of reduction in tumor cell abundance. Analysis of the scRNA-seq data revealed transcriptional changes in four cellular programs common among the multiple myeloma (MM) patients. These included unfolded protein response (UPR), metabolic-associated, stress-associated, and immune reactive programs. Moreover, MM patients that did not respond to the PI treatment had the lowest score in the immune reactive programs, and tumor cells with lower immune reactive programs exhibited immune escape phenotype. By further characterizing specific cell types in the tumor microenvironment, they identified a transcription factor YBX1 (Y-box binding protein-1), which was upregulated in myeloma cells with low immune reactive status. Overall, these findings imply that elevated levels of YBX1 may induce a "cold" tumor immune phenotype, marked by decreased levels of infiltrating leukocytes, attenuated immune cell activation, and the emergence of immunosuppressive signaling. This underscores the potential of YBX1 as a promising therapeutic target.
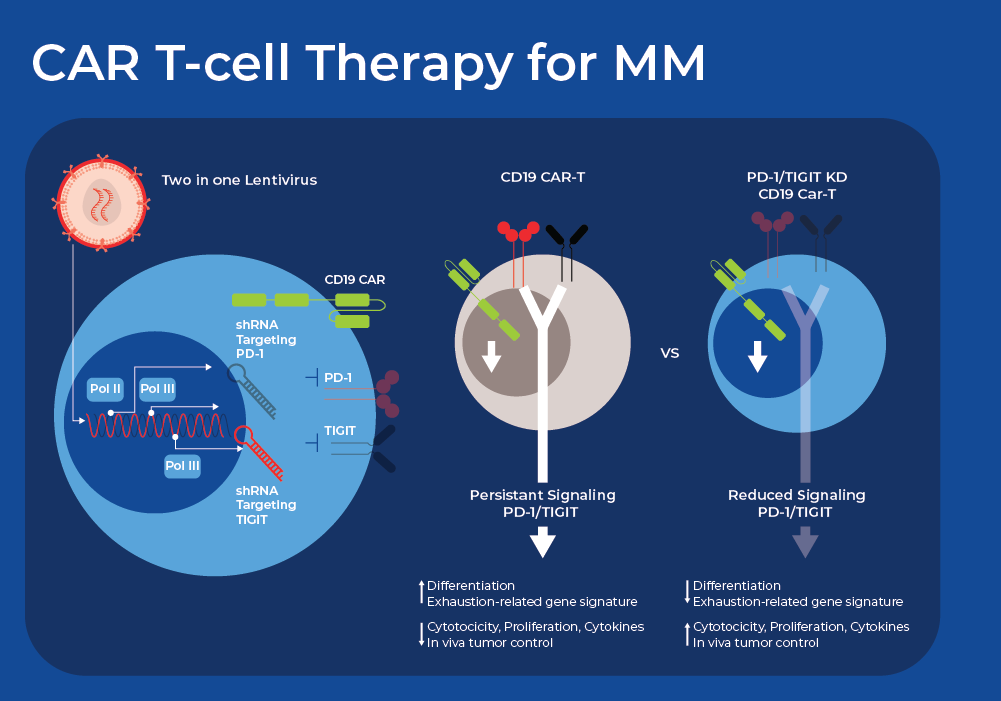
RNA Therapeutics and CAR T-cell Therapy for Treatment of Multiple Myeloma
Although traditional treatments like surgery, chemotherapy, and radiation therapy are still the first round of treatment for most cancers, new immune based therapies are showing great promise. A particularly promising immunotherapy is Chimeric Antigen Receptor (CAR) T-cell therapy. This therapy involves withdrawing a patient's blood, separating their T-cells, genetically engineering those T-cells to express Chimeric Antigen Receptors (CARs), then infusing these CAR-expressing T-cells back into the patient. The CARs are fusion proteins engineered to selectively target antigens present on the surfaces of cancer cells, thus eradicating them.9 Since 2017, the Food and Drug Administration (FDA) has approved six CAR T-cell therapies that all target various forms of blood cancers, including lymphomas, certain types of leukemia, and most recently, multiple myeloma.
Despite the promising initial outcomes, recent long-term clinical follow-up shows primary resistance affects 10-20% of patients, and relapse occurs in about 30-50% of patients.10 One common reason for CAR T failure is high expression of multiple inhibitory immune checkpoint receptors (ICRs). Researchers are now exploring innovative ways using small non-coding RNAs (sncRNAs) to down regulate or silence some of these ICRs to improve the outcomes of CART therapy. There are a wide variety of sncRNAs involved in gene silencing which can be harnessed by researchers. Short hairpin RNA, also known as a small hairpin RNA (shRNA or Hairpin Vector), is an engineered RNA molecule featuring a compact hairpin structure shRNA is processed in the cells to form siRNA that can suppress target gene expression through RNA interference (RNAi).
Researchers recently demonstrated the use of a dual shRNA-based strategy that simultaneously downregulates twoICRs in CAR T-cells. They tested distinct combinations of ICR downregulation and found varying effects on the functionality of CAR-T cells, with some combinations proving deleterious. However, they found that simultaneous downregulation of PD-1 and TIGIT (T cell immunoreceptor with immunoglobulin [Ig] and ITIM domains) greatly improved the performance of CAR T-cells derived from both healthy donor and NHL patient T cells. Crucially, functional and phenotypic analyses indicated that the reduction of PD-1 enhanced short-term effector function, while the decrease in TIGIT expression primarily sustained a less differentiated or exhausted state. This offers a potential mechanism for the observed synergy. CAR T-cells with PD-1 and TIGIT downregulation, derived from T-cells of patients with diffuse large B cell lymphoma, also exhibit robust antitumor activity and significantly enhanced persistence in vivo. Currently, the efficacy and safety of CD19-targeting CAR T-cells with PD-1 and TIGIT downregulation are under evaluation in adult patients with relapsed or refractory large B-cell lymphoma.11
Another avenue for improving the outcome of CART therapy is modulating the gut microbiome. A significant body of research already shows that the gut microbiome composition, probiotics, and prebiotics can improve the outcome of immunotherapy, such as anti-CTLA-4 and anti-PD-1 therapies.12,13 Research is revealing a similar effect of the gut microbiome on the efficacy of CART therapy. For example, metagenomic sequencing of patients undergoing CART therapy showed that the abundance of Bifidobacterium longum, and peptidoglycan biosynthesis, strongly correlated with response to CART and long term survival.14 Moreover, microbiome-based machine-learning algorithms robustly segregated long-term responders from non-responders with Bacteroides, Ruminococcus, Eubacterium, and Akkermansia being the most important in determining CART responsiveness.