Plasma/Serum RNA Purification Kits
For rapid and simple purification of circulating RNA and Exosomal RNA from plasma/serum samples
Plasma/Serum RNA Purification Kits
For rapid and simple purification of circulating RNA and Exosomal RNA from plasma/serum samples
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Isolate all sizes of circulating and exosomal RNA, including microRNA
- Versatile plasma/serum input ranges
- No phenol extractions
- No carrier RNA
- Bind and elute all RNA irrespective of size or GC content, without bias
- Concentrate circulating RNA and exosomal RNA into a flexible elution volume
- High quality, purified RNA is suitable for a variety of downstream applications, including Small RNA Sequencing. Find out more information on Norgen's NGS services
- Compatible with Streck Cell-Free RNA BCT® Tubes
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
These kits provide a fast, reliable and convenient method to purify and concentrate high quality, high purity and inhibitor-free cell-free circulating and exosomal RNA using a convenient spin column method. These kits can purify RNA from fresh or frozen serum or plasma samples prepared from blood collected on either EDTA or Citrate. Plasma samples prepared from blood collected on heparin should not be used, as heparin can significantly interfere with many downstream applications such as RT-PCR. The purified plasma/serum RNA is fully compatible with all downstream applications including PCR, qPCR, methylation-sensitive reverse transcription qPCR, reverse transcription PCR, Northern blotting, RNase protection and primer extension, expression array assays, and NGS.
Background
Plasma/Serum cell-free circulating RNA or exosomal RNA has the potential to provide biomarkers for certain cancers and disease states. Exosomes are 40 - 150 nm membrane vesicles, which are secreted by most cell types. Exosomes can be found in saliva, blood, urine, amniotic fluid and malignant ascitic fluids, among other biological fluids. Evidence has been accumulating recently that these vesicles act as cellular messengers, conveying information to distant cells and tissues within the body. These exosomes may play a functional role in mediating adaptive immune responses to infectious agents and tumours, tissue repair, neural communication and transfer of pathogenic proteins. For this reason exosomal RNAs may serve as biomarkers for various diseases including cancer. As the RNA molecules encapsulated within exosomes are protected from degradation by RNAses they can be efficiently recovered from biological fluids, such as plasma or serum.
Plasma/Serum RNA Purification Mini Kit
This kit can purify RNA from fresh or frozen serum or plasma samples prepared from blood collected on either EDTA or Citrate, from volumes ranging from 50 µL to 200 µL. The purified plasma/serum RNA is eluted in a flexible final volume of 10 µL to 25 µL.
Plasma/Serum RNA Purification Midi Kit
This utilizes a two column method, and can purify RNA from fresh or frozen serum or plasma samples prepared from blood collected on either EDTA or Citrate, from volumes ranging from 250 µL to 1.5 mL. The first column will handle the large volume input of bodily fluids that is followed by a concentration on a mini column for a final elution of 50 µL to 100 µL.
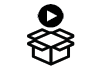
Plasma/Serum RNA Purification Maxi Kit
This kit can purify RNA from fresh or frozen serum or plasma samples prepared from blood collected on either EDTA or Citrate, from volumes ranging from 2 mL to 5 mL. The first column will handle the large volume input of bodily fluids that is followed by a concentration on a mini column for a final elution of 50 µL to 100 µL.
Details
Supporting Data
Figure 1. Purification of circulating RNA from different plasma volumes. Norgen's Plasma/Serum RNA Purification Mini Kit was used to purify circulating RNA from 50 µL, 100 µL and 200 µL plasma prepared from blood collected on EDTA. Three microlitres of the purified RNA was then used as the template in RT-qPCR reactions to detect miR-21 (Figure 1A) and the housekeeping 5S rRNA transcript (Figure 1B). The relative amount of both the miR-21 (Figure 1A) and the 5S rRNA transcript (Figure 1B) is linearly increasing with increasing the sample input volume.
Figure 2. Eluting purified circulating RNA into different elution volumes. Norgen’s Plasma/Serum RNA Purification Mini Kit was used to purify circulating RNA from 200 µL plasma prepared from blood collected on EDTA and eluted in 10 µL, 15 µL, and 25 µL. Three microlitres of the purified RNA was then used as the template in RT-qPCR reactions to detect miR-21 (Figure 2A) and the housekeeping 5S rRNA transcript (Figure 2B). The relative amount of both the miR-21 (Figure 2A) and the 5S rRNA transcript (Figure 2B) is increasing with increasing the elution volume indicating the efficient concentration of the plasma circulating RNA in a very low elution volume.
Figure 3. Effective and consistent detection of miRNA from plasma. Norgen's Plasma/Serum RNA Purification Mini Kit can effectively isolate miRNA from plasma. Circulating miRNA was isolated from 200 µL plasma using Norgen's Plasma/Serum RNA Purification Mini Kit, competitor Q's kit and competitor E's kit. Circulating miRNA was isolated from 600 µL using competitor A's kit. Stem loop RT-qPCR using primers specific to miR-21 was performed. In brief, 1 microliter of the 15 µL RNA purified using Norgen's Plasma/Serum RNA Purification Mini Kit, competitor Q's kit and 3.3 microliters of the 50 µL purified RNA using competitor E's kit and competitor A's kit was then subjected to a 20 µL reverse transcription using miR-21 stem-loop reverse primer. Three microliters of the reverse transcription was used in a 20 µL real-time PCR reaction with primers to detect the human miR-21. Norgen's Plasma/Serum RNA Purification Mini Kit showed the most consistent and the highest recovery of the miR-21 transcripts as compared to the other isolation methods. The recovery of the miRNA from 200 µL plasma using Norgen's kit was higher than that recovered from RNA purified from 600 µL using competitor A's kit.
Figure 4. Small RNA Sequencing from as little as 50 µL of Plasma. Norgen Biotek has developed an effective pipeline for small RNA sequencing from small volumes of plasma/serum. RNA can be effectively and consistently recovered from as little as 50 µL of plasma using Norgen's patented sample preparation technology (example shown here with Plasma/Serum RNA Purification Mini Kit, Cat. 55000). Panel A shows that the number of microRNA detected from 50 or 200 µL of plasma was almost identical to that of 4 mL. Panel B is a Venn diagram showing that of the microRNAs identified, the majority are detected in all volumes of plasma input from 50 µL to 4 mL. In fact, the scatter plots in Panel C show that the relative expression level of each microRNA detected was highly correlated between 50 or 200 µL of plasma and 4 mL plasma.
Figure 5. High Degree of Overlapping of miRNA profile between Urine and Plasma. Plasma and mid-stream urine was collected from three different healthy individuals. RNA was isolated from 20 mL of each urine sample using Norgen's Urine Exosomal RNA Purification Kit (Cat. 47200) and 200 µL of each plasma sample using Norgen's Plasma/Serum RNA Purification Mini Kit (Cat. 55000). Small RNA Libraries were then generated using an Illumina TruSeq Small RNA Library Preparation Kit and subsequently sequenced on an Illumina MiSeq system. The list of mapped miRNAs from plasma and urine from the same individual was then compared. The Venn diagrams showed that the miRNA profiles have high degree of overlapping between urine and plasma within each individual.
Figure 6. Increased Diversity of Other Small RNA Species (Piwi-Interacting) in Urine. Plasma and mid-stream urine was collected from three different healthy individuals. RNA was isolated from 20 mL of each urine sample using Norgen's Urine Exosomal RNA Purification Kit (Cat. 47200) and 200 µL of each plasma sample using Norgen's Plasma/Serum RNA Purification Mini Kit (Cat. 55000). Small RNA Libraries were then generated using an Illumina TruSeq Small RNA Library Preparation Kit and subsequently sequenced on an Illumina MiSeq system. The above chart showed that the relative proportion of piwi-interacting RNA (piRNA) was consistently higher in urine.
Figure 7. Better Diversity of miRNA Detected from HeLa Cells using Illumina Small RNA Next Gen Sequencing. Norgen's Total RNA Purification Kit isolates miRNA from HeLa cells with better diversity than a leading competitor. Total RNA including miRNA was isolated from 1 million HeLa cells using Norgen's Total RNA Purification Kit and Competitor Q's leading miRNA Kit, and was applied to Illumina Small RNA Next Gen Sequencing on a MiSeq sequencer. Panel A showed that Norgen’s Total RNA Purification Kit recovers a higher number of miRNAs than the competitor. In particular, the higher diversity is achieved with a faster and simpler procedure in as little as 20 minutes of RNA sample preparation time without the use of phenol. Panel B is a scatter plot of the average RPM (reads per million) of the miRNAs detected to compare Norgen and the competitor’s recovery of miRNA. Norgen’s Total RNA Purification Kit recovered a significantly higher number of miRNAs that have higher RPM, as summarized in the graph insert as well as in Panel C.
Figure 8. Purification of cell-free circulating RNA and exosomal RNA from different plasma volumes. Norgen’s Plasma/Serum RNA Purification Midi Kit (Cat# 56100) was used to purify cell-free circulating and exosomal RNA from 0.25 mL, 0.75 mL and 1.5 mL plasma prepared from blood collected on citrate as an anticoagulant. Two microlitres of the purified RNA was then used as the template in RT-qPCR reactions to assess the amplification of the (A) housekeeping 5S rRNA transcript and (B) miR-21. The average Ct value for both the 5S rRNA transcript and the miR-21 is linearly decreasing with increasing the sample input volume.
Figure 9. Linearity of RNA purified from increasing plasma volumes using Norgen’s Plasma/Serum RNA Purification Midi Kit. Norgen’s Plasma/Serum RNA Purification Midi Kit (Cat# 56100) was used to purify RNA from 0.25 mL, 0.7 mL and 1.5 mL plasma prepared from blood collected on citrate as an anticoagulant. Two microlitres of the purified RNA was then used as the template in RT-qPCR reactions to assess the linearity of (A) the housekeeping 5S rRNA transcript and (B) miR-21 from the different plasma volumes. Norgen’s Plasma/Serum RNA Purification Midi Kit was able to recover 97% of both the 5S rRNA transcript and the miR-21 transcript from 0.75 mL plasma relative to the amount that is present in 0.35 mL plasma. Moreover, 95% of the 5S rRNA transcript and the miR-21 was recovered from 1.5 mL plasma relative to the amount that is present in 0.75 mL plasma.
Figure 10. Determination of the amount of inhibition present in plasma RNA samples when detecting the human 5S transcript and miR-21. RNA was isolated from 0.25 mL, 0.75 mL and 1.5 mL plasma using Norgen’s Plasma/Serum RNA Purification Midi Kit (Cat# 56100). Increasing volumes of the elution (2, 4 and 8 µL) were used in a 20 µL reverse transcription reaction followed by qPCR amplification reaction to observe any decrease in Ct value. An increase in Ct values with increasing amount of template would be a clear indication of PCR inhibitors present in the sample. An increase in the PCR input volume used as a template in the reverse transcription reaction did not affect the Ct value generated from the qPCR amplification for both (A) 5S rRNA transcript and (B) miR-21. In fact the Ct values tend to decrease with increasing the PCR input volume indicating that RNA purified from plasma using Norgen’s kit is free of the common inhibitors usually present in plasma.
Figure 11. Purification of cell-free circulating RNA and exosomal RNA from different plasma volumes. Norgen’s Plasma/Serum RNA Purification Maxi Kit (Cat# 56200) was used to purify cell-free circulating and exosomal RNA from 1.5 mL, 3 mL and 5 mL plasma prepared from blood collected on citrate as an anticoagulant. Two millilitres of the purified RNA was then used as the template in RT-qPCR reactions to assess the amplification of (A) the housekeeping 5S rRNA transcript and (B) miR-21. The average Ct value for both the 5S rRNA transcript and the miR-21 is linearly decreasing with increasing the sample input volume.
Figure 12. Linearity of RNA purified from increasing plasma volumes using Norgen’s Plasma/Serum RNA Purification Maxi Kit. Norgen’s Plasma/Serum Cell-Free Circulating and Exosomal RNA Purification Maxi Kit (Cat# 56200) was used to purify RNA from 1.5 mL, 3 mL and 5 mL plasma prepared from blood collected on citrate as an anticoagulant. Two microlitres of the purified RNA was then used as the template in RT-qPCR reactions to assess the linearity of (A) the housekeeping 5S rRNA transcript and (B) miR-21 from the different plasma volumes. Norgen’s Plasma/Serum RNA Purification Maxi Kit was able to recover 92% of the 5S rRNA transcript from 3 mL plasma relative to the amount that is present in 1.5 mL plasma. Moreover, 90% of the 5S rRNA transcript was recovered from 5 mL plasma relative to the amount that is present in 3 mL plasma. As for miR-21, Norgen’s Plasma/Serum RNA Purification Maxi Kit was able to recover 91% of miR-21 from 3 mL plasma relative to the amount that is present in 1.5 mL plasma. Furthermore, 90% of miR-21 was recovered from 5 mL plasma relative to the amount that is present in 3 mL plasma.
Figure 13. Determination of the amount of inhibition present in plasma RNA samples when detecting the human 5S transcript and miR-21. DNA was isolated from 1.5 mL, 3 mL and 5 mL plasma using Norgen’s Plasma/Serum RNA Purification Maxi Kit (Cat# 56200). Increasing volumes of the elution (2, 4 and 8 µL) were used in a 20 µL reverse transcription reaction followed by qPCR amplification reaction to observe any decrease in Ct value. An increase in Ct values with increasing amount of template would be a clear indication of PCR inhibitors present in the sample. An increase in the PCR input volume used as a template in the reverse transcription reaction did not affect the Ct value generated from the qPCR amplification for both (A) 5S rRNA transcript and (B) miR-21. In fact the Ct. values tend to decrease with increasing the PCR input volume indicating that RNA purified from plasma using Norgen’s kit is free of the common inhibitors usually present in plasma.
|
Kit Specifications
|
|
| Sample Volume Range |
50 to 200 μL
|
|
Anti-coagulant (for Plasma)*
|
EDTA or Citrate
|
| Size of RNA Purified |
All sizes, including
small RNA (< 200 nt) |
| Minimum Elution Volume |
10 μL
|
| Maximum Elution Volume | 25 μL |
| Time to Complete 10 Purifications |
15-20 minutes
|
| Average Yield** |
Variable depending on specimen
|
*This kit is suitable for the isolation of RNA from fresh or frozen serum or plasma prepared from blood collected on either EDTA or Citrate. Plasma samples prepared from blood collected on heparin should not be used as heparin can significantly interfere with many downstream applications such as RT-PCR.
**Please check page 7 for Average Plasma/Serum Yields and Common RNA Quantification Methods.
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 2 years after the date of shipment. It is recommended to warm Lysis Buffer A for 20 minutes at 60°C if any salt precipitation is observed.
| Kit Components | Cat. 55000 (50 preps) | Cat. 56100 (20 preps) | Cat. 56200 (10 preps) |
|---|---|---|---|
| Lysis Buffer A | 2 x 20 mL | 100 mL | 1 x 130 mL 1 x 30 mL |
| Wash Solution A | 18 mL | 1 x 38 mL 1 x 18 mL |
38 mL |
| Elution Solution A | 6 mL | 6 mL | 6 mL |
| Elution Buffer F | - | 15 mL | 15 mL |
| Micro Spin Columns | 50 | - | - |
| Mini Spin Columns | - | 20 | 10 |
| Midi Spin Columns | - | 20 | - |
| Maxi Spin Columns | - | - | 10 |
| Collection Tubes | 50 | 20 | 10 |
| Elution Tubes (1.7 mL) | 50 | 20 | 10 |
| Product Insert | 1 | 1 | 1 |
Documentation
FAQs
Mini, Midi, Maxi
Citations
| Title | A set of circulating microRNAs belonging to the 14q32 chromosome locus identifies two subgroups of individuals with recent-onset type 1 diabetes |
| Citation | Cell reports medicine 2024. |
| Authors | Guido Sebastiani, Giuseppina Emanuela Grieco, Marco Bruttini, ..., Chantal Mathieu, Francesco Dotta, INNODIA investigators |
| Title | Evaluation of miR-148a-3p and miR-106a-5p as Biomarkers for Prostate Cancer: Pilot Study |
| Citation | Genes 2024. |
| Authors | Roxana Andra Coman 1,†ORCID,Vlad Horia Schitcu 2,†ORCID,Liviuta Budisan 3ORCID,Lajos Raduly 3ORCID,Cornelia Braicu 3ORCID,Bogdan Petrut 1ORCID,Ioan Coman 1,Ioana Berindan-Neagoe 3,*ORCID andNadim Al Hajjar 4,5 |
| Title | Minimally invasive determination of PDAC subtype and therapy-induced subtype switch by means of circulating cell-free RNA |
| Citation | Preprint Research Square 2024. |
| Authors | Smiths Lueong1 ORCID Email Martin Metzenmacher2 Marija Trajkovic-Arsic2 Phyllis F. Y. Cheung2 Timm M. Reißig2 Nils von Neuhoff3 O'Kane Grainne4 Steven Gallinger4 Stephanie Ramotar4 Anna Dodd4 Jennifer J Knox4 Alexander Muckenhuber5 Volker Kunzmann6 Peter A. Horn7 Jörg D. Hoheisel8 Jens Thomas Siveke2 |
| Title | Overexpression of circular RNA hsa_circ_0008621 facilitates colorectal cancer progression and predicts poor prognosis |
| Citation | Annals of gestroenterological Surgery 2024. |
| Authors | Xiaohu Zhou | Lei Wu | Chunyan Tian |
| Title | Plasma-derived exosomal miR-326, a prognostic biomarker and novel candidate for treatment of drug resistant pediatric acute lymphoblastic leukemia |
| Citation | Nature Scientific Reports 2024. |
| Authors | Neda Saffari,Soheila Rahgozar,Elaheh Faraji &Fikrettin Sahin |
| Title | Bovine embryos release extracellular vesicles with differential miRNA signature during the compaction and blastulation stages |
| Citation | Reproductive Biology 2023. |
| Authors | Bárbara Melo-Báez, Edwin A. Mellisho, Yat S. Wong, Joel Cabezas, Diego Caamaño, Constanza Aguilera, Gonzalo Riadi, Fidel O. Castro, Lleretny Rodriguez-Alvarez |
| Title | Early transcriptomic signatures and biomarkers of renal damage due to prolonged exposure to embedded metal |
| Citation | Cell Biology and Toxicology 2023. |
| Authors | Yuan Wen, Ivan J. Vechetti, Dongliang Leng, Alexander P. Alimov, Taylor R. Valentino, Ziaouhua D. Zhang, John J. McCarthy & Charlotte A. Peterson |
| Title | Host miRNAs as biomarkers of SARS-CoV-2 infection: a critical review |
| Citation | Sensors & Diagnostics 2023. |
| Authors | Kato Pollet, Nathalie Garnier, Sabine Szunerits, Annemieke Madder, Didier Hober and Ilka Engelmann |
| Title | Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy |
| Citation | Nature biomedical engineering 2023. |
| Authors | Yi You, Yu Tian, Zhaogang Yang, Junfeng Shi, Kwang Joo Kwak, Yuhao Tong, Andreanne Poppy Estania, Jianhong Cao, Wei-Hsiang Hsu, Yutong Liu, Chi-Ling Chiang, Benjamin R. Schrank, Kristin Huntoon, DaeYong Lee, Ziwei Li, Yarong Zhao, Huan Zhang, Thomas D. Gallup, JongHoon Ha, Shiyan Dong, Xuefeng Li, Yifan Wang, Wen-Jing Lu, Eman Bahrani, Ly James Lee, Lesheng Teng, Wen Jiang, Feng Lan, Betty Y. S. Kim & Andrew S. Lee |
| Title | Uncovering the expression of circPVT1 in the extracellular vesicles of acute myeloid leukemia patients |
| Citation | Biomedicine & Pharmacotherapy 2023. |
| Authors | Martina Ghetti , Ivan Vannini , Maria Teresa Bochicchio , Irene Azzali , Lorenzo Ledda , Giovanni Marconi , Mattia Melloni , Francesco Fabbri , Michela Rondoni , Roberta Chicchi , Davide Angeli , Andrea Ghelli Luserna di Rorà , Barbara Giannini , Irene Zacheo , Rino Biguzzi , Francesco Lanza , Giovanni Martinelli , Giorgia Simonetti |