Movember started 20 years ago in a pub in Australia when two mates (aka friends), Travis Garone and Luke Slattery, decided they wanted to bring back the "stash" and do good at the same time. They were inspired by a friend's mother who was fundraising for breast cancer, and decided to raise funds for men's health and prostate cancer. Trav designed the first "Movember" logo, and they sent around an email titled "Are you man enough to be my man?" They found 30 guys willing to take up the challenge, and charged 10 dollars to "Grow a Mo". The next year they up-ed their game and started a fundraising campaign for the Prostate Cancer Foundation of Australia (PCFA), and provided them with the largest single donation the PCFA had ever received. Now, Movember has morphed into an international movement that last year raised 115 million dollars globally. During their research into men's health, they realized that depression is also a major health issue affecting men. The three main goals of the movement are now: 1) Mental Health and Suicide Prevention, 2) Prostate Cancer, 3) Testicular Cancer. This blog will focus on recent research that is uncovering the intricate link between the gut microbiome and our mental health.
The Movember website presents stark statistics stating that
GLOBALLY, ON AVERAGE, 1 MAN DIES BY SUICIDE EVERY MINUTE OF EVERY DAY.

The Gut Microbiome Influences Mental Health
The idea that the gut is central to health is an ancient concept predominant in Traditional Chinese Medicine, Ayurvedic Medicine, and Hippocratic Medicine. "All disease begins in the gut" is a quote attributed to the Ancient Greek physician Hippocrates nearly 2500 years ago. Interest in the importance of the gut was re-ignited in the early 2000's as sequencing prices dropped and computational advances began to assemble a picture of the microbial diversity within the gut. This soon led to discoveries about the relationship between the gut microbiome and a variety of diseases, ranging from obesity, type 2 diabetes, cardio-metabolic diseases,1 neurodegenerative diseases,2,3 and even discoveries of gut microbiota modulating responses to cancer immunotherapy.4 More recently, a new area has opened up that focuses on the impact of the gut microbiome on the brain, and even more specifically, the gut and mental health.
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
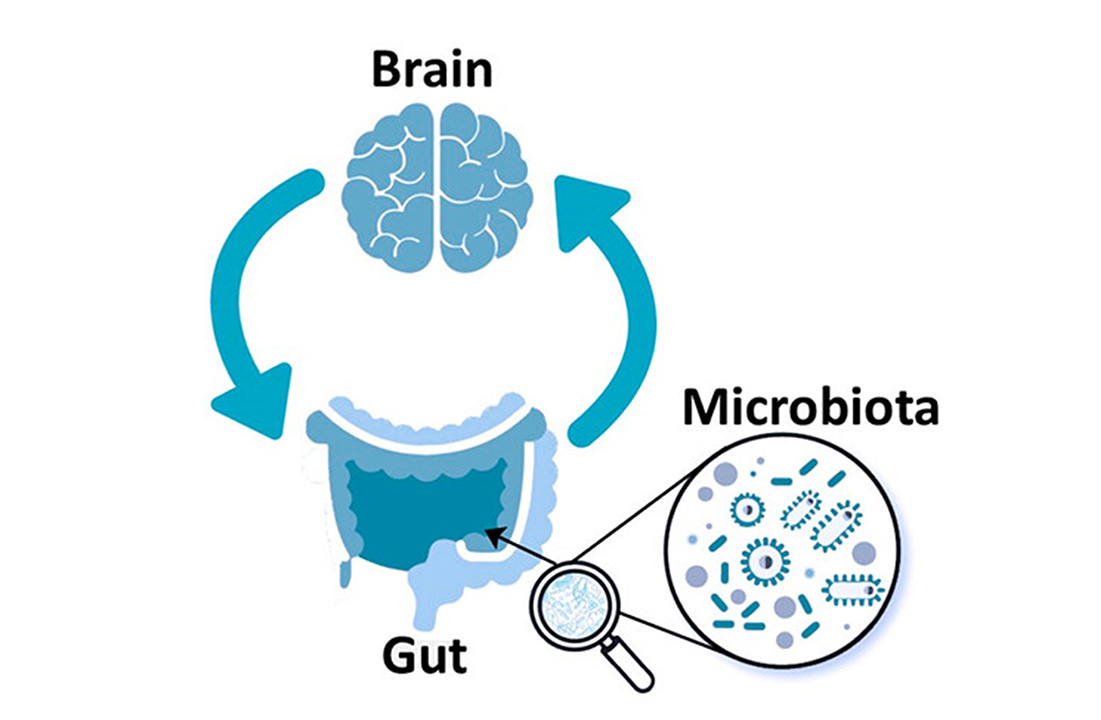
Multiple Pathways Along the Gut-Brain Axis
Bacterial Metabolites as Signaling Molecules
A variety of substances secreted by microbes into the gut can directly reach the brain through blood vessels. These compounds include short-chain fatty acids (SCFAs), neuro-active compounds, and even cell-wall components such as LPS. In 2016, as a product of experiments using mouse models of Parkinson's Disease (PD), researchers identified that short-chain fatty acid signaling modulated microglia and enhanced PD pathophysiology. Moreover, researchers found that transferring microbes taken from the guts of people with Parkinson's to mice induced motor dysfunction in the mice. Motor dysfunction also occurred if the mice were given just the short-chain fatty acid metabolites from Parkinson's patients.5
More recently, researchers have shown that ammonia produced in the gut can help buffer stress in the host through replenishing GABA. Researchers showed that microbial nitrogen metabolism is important in host stress vulnerability by maintaining brain glutamine availability. Moreover, through the introduction of a urease-producing strain, Streptococcus thermophilus, they reversed depression-like behaviours in the mice. These findings could also lead to potential therapies as they found that ammonium chloride (NH4Cl) rescued behavioural abnormalities and GABAergic deficits in mouse models of depression.6
Microbial Interaction via the Vagus Nerve
Early reports on the effect of stress on the gut revealed a connection between the gut and the brain, and we are now learning that part of this connection may be through the vagus nerve. Early studies on the relationship between stress and the gut microbiome showed that germ-free mice had more detrimental reactions to stress than non-germ-free mice. This was followed up by work that showed that stress due to early separation of rat pups from their mothers induced long-term changes in their gut microbiome.7 Finally, work started to lead towards a mechanism when research showed that mice that received supplements of Lactobacillus rhamnosus JB-1 produced less stress-induced corticosterone and displayed fewer anxiety and depression related behaviors. The probiotic supplementation also altered the production of a protein receptor in the brain that binds to the neurotransmitter ℽ-aminobutyric acid (GABA). However, mice with a severed vagus nerve did not benefit from the probiotic supplementation, suggesting that the effects were being transmitted through the vagus nerve.8
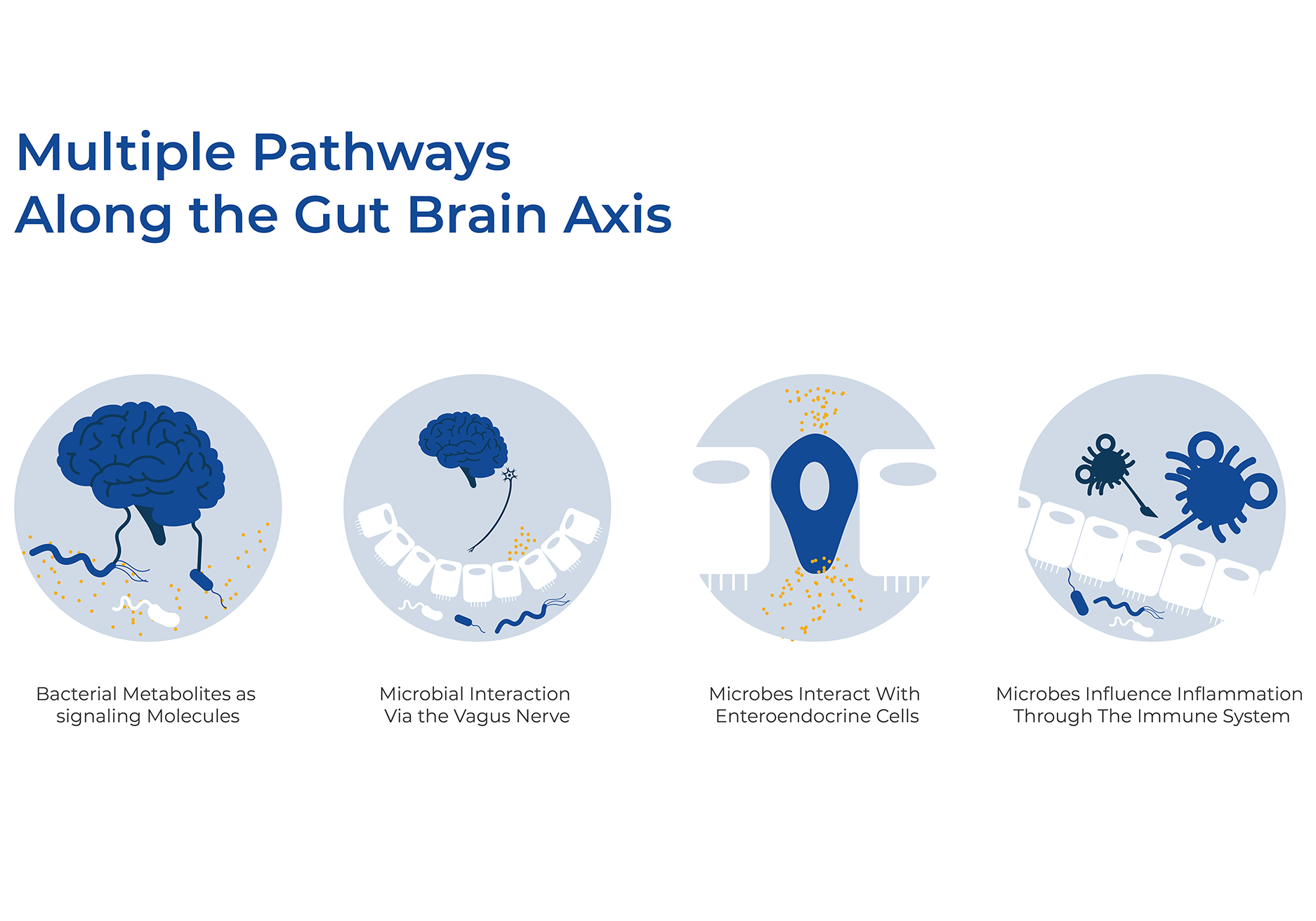
Microbes interact with Enteroendocrine Cells
Microbes also interact with enteroendocrine cells (EECs) in the gut lining to send hormones throughout the body. EECs sense changes in luminal microorganisms via microbial metabolites, and communicate with host body systems via neuroendocrine molecules such as serotonin or GLP-1. A recent transcriptome-wide association study of 23 tissues revealed shared susceptibility genes between Crohn's disease, ulcerative colitis, and schizophrenia. Analysis of bulk and single-cell RNA-sequencing data showed that gene expression was strongest in neurons and enteroendocrine cells in the colon and rectum, indicating a possible link to the gut-brain-axis.9
Gut Microbes Impact Inflammation
More indirectly, gut microbiota influence immune cells and inflammation pathways, which can affect the brain. For more than 20 years, inflammation has been associated with depression. One demonstration of this is the fact that several antidepressants have been shown to reduce the endogenous production of pro-inflammatory cytokines, thus modifying immune reactivity in the brain. There is also evidence in the pathogenesis of depression that there is increased bacterial translocation due to the disruption of tight junctions and barrier integrity of the GI tract. Moreover, recent evidence indicates that microbiota dysbiosis is associated with the development of several chronic inflammatory disorders. More specifically, several gut microbial taxa have been shown to stimulate the anti-inflammatory cytokine, including IL-10 and downregulate inflammatory cytokines. Evidence from rodent experiments also supports a link between microbiota and depression through inflammatory markers. Furthermore, many clinical studies have shown a decrease in depressive symptoms following probiotic treatment.10,11
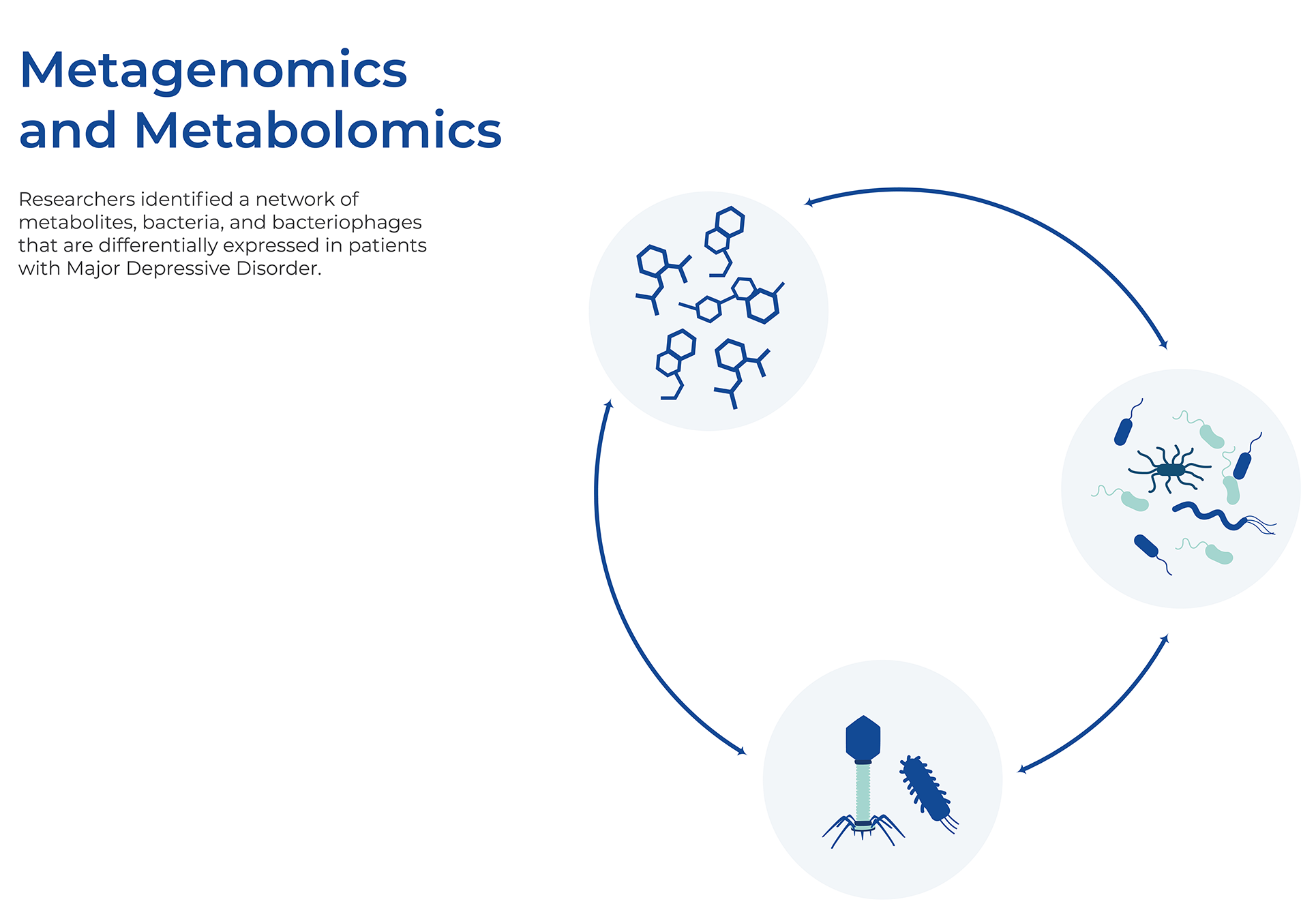
Metagenomics and Metabolomics Links the Gut Microbiome to MDD
Researchers recently analyzed whole-genome shotgun metagenomic sequences and combined that with untargeted metabolomic data from over 300 individuals with Major Depressive Disorder (MDD) and paired Healthy Controls (HCs). They identified a network of bacteria, bacteriophages, and metabolites that were differentially expressed between patients with MDD and HCs. Patients with MDD were mainly characterized by increased abundance of the genus Bacteroides, and decreased abundance of the genera Blautia and Eubacterium. There was concordance between the differentially expressed genes and fecal metabolites as both data sets point to differential expression of amino acids (ℽ-aminobutyrate, phenylalanine, and tryptophan). Together, these findings may lead to the development of probiotics or postbiotics and easier diagnosis as they identified a combinatorial marker panel that robustly discriminates MDD from HC individuals.12
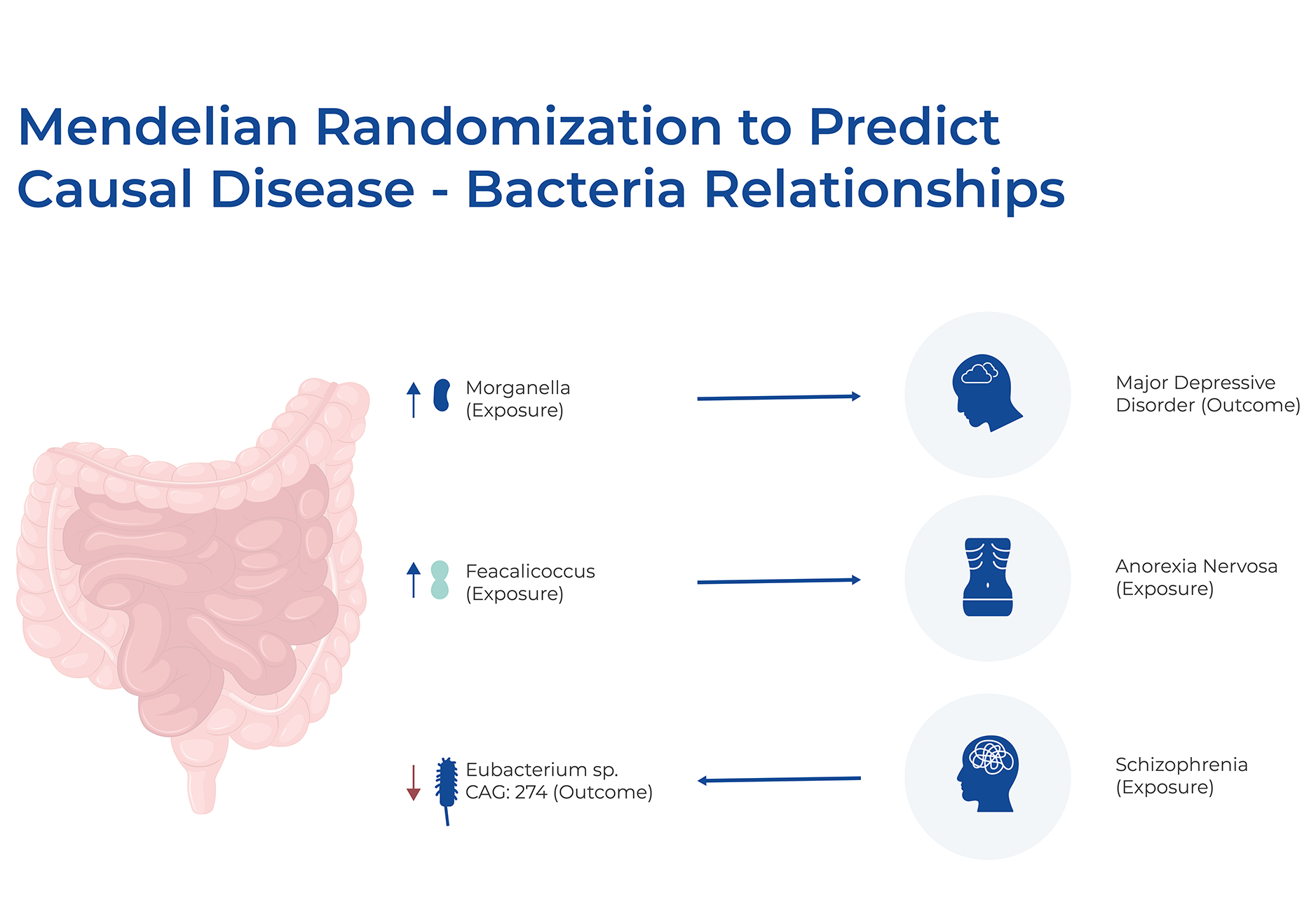
Combining Host and Microbiome Sequencing
Long-term cohort studies that collect multiple datasets can provide a wealth of information. Recently, one such cohort study, FINRISK 2002, analyzed matching human genotypes and shotgun fecal metagenomes for almost 6,000 individuals. They combined these genome-wide associations with extensive health and dietary data to predict incident disease linked to gut microbial variation. They had health records for up to 16 years after the initial microbiome samples, which allowed for observational validations of predicted effects. Using Mendelian randomization, they predicted 96 causal effects in both microbe-to-disease and disease-to-microbe directions. Of these effects, 34 indicated that microbial abundances can be used to predict a disease, and a large proportion of those diseases were psychiatric and neurological. For example, an increased abundance of Faecalicoccus may have a causal effect on anorexia nervosa, and an increased abundance of Morganella and Raoultella are predicted to have causal effects on MDD. Interestingly, when Mendelian randomization was performed in the reverse direction, using disease risk as an exposure and microbial level as an outcome, most predicted causal effects involved autoimmune and inflammatory diseases.13
Consider the Microbiome in Any Research Question
Any field of Human Health research should take into account the effect of the microbes that live within and on us. Whatever your field of research is, from psychology to immunology, and whether your expertise is in metabolomics, proteomics, biochemistry, or human genetics, you should consider including analysis of RNA or DNA extracted from fecal samples in your study design.
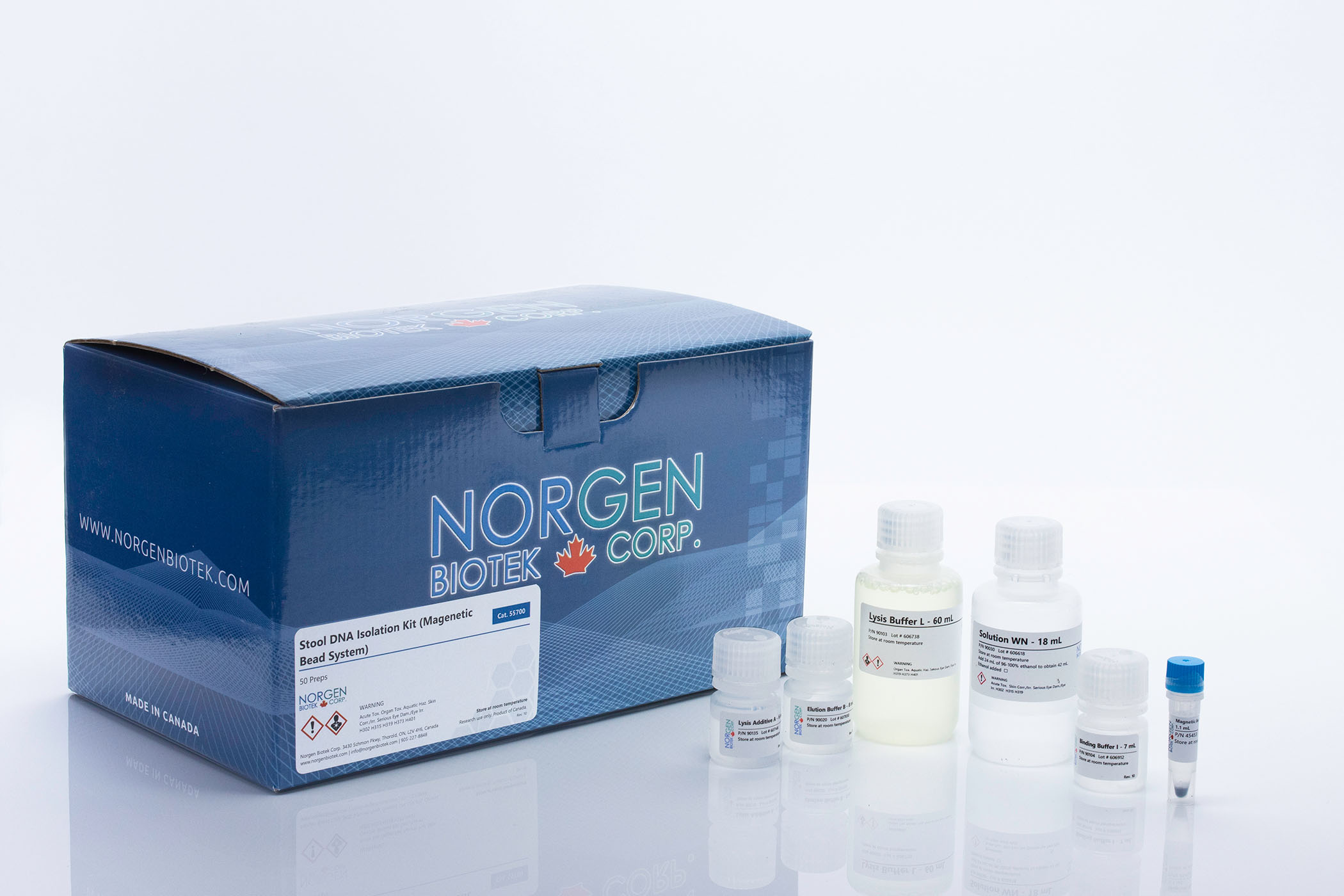
Norgen's Stool Microbiome Workflow
Norgen has a complete workflow for studying the fecal microbiome, encompassing collection, extraction of both DNA and RNA, and sequencing. Norgen has a variety of sample storage and preservation tubes, including the patented Stool Nucleic Acid Collection and Preservation tubes, either CE-IVD marked for diagnostic purposes (Cat. Dx45660) or for research purposes (Cat. 45660). We also have Fecal collection mini tubes (Cat. 27650) for mouse or other rodent microbiome studies, and a Fecal Swab Collection and Preservation System (Cat. 45670-B). Then there are Norgen's Stool DNA extraction kits using spin columns (Cat. 27600, 65600) or magnetic beads (Cat. 55700, Cat. 63100) for more high throughput approaches. These magnetic bead protocols can be automated on the Hamilton Vantage, or similar machines, or magnetic rod-based systems. Or if you would like to investigate transcriptomics, there is Norgen's Stool Total RNA Purification Kit (Cat. 49500), or the Stool Nucleic Acid Isolation Kit (Cat. 45600), to extract both DNA and RNA. You can also prepare your own 16S or ITS Metagenomic libraries for sequencing. Lastly, Norgen offers Metagenomic Sequencing Services.